Before initiating Mitotane, evaluate pelvic ultrasound in premenopausal women, liver functions tests and complete blood count.
Mitotane is a hazardous drug. Advise caregivers to wear disposable gloves when handling Mitotane tablets.
• The recommended initial dose of Mitotane is 2000 mg to 6000 mg orally, in three or four divided doses per day.
• Monitor mitotane plasma levels and increase the dose based on patient tolerance and clinical response incrementally to achieve a mitotane plasma level of 14 to 20 mg/L, or as tolerated. Consider monitoring mitotane plasma levels every 2 weeks after starting treatment and after each dose adjustment.
• The target plasma level is usually reached within a period of 3 to 5 months.
• Monitor mitotane plasma levels periodically (e.g., monthly). Severe neurotoxicity may occur with levels > 20 mg/L.
• In case of mitotane plasma levels above 20 mg/L without toxicities, consider reducing the dose by 50 to 75%.
• Monitor mitotane plasma levels regularly (e.g., every two months) after discontinuation of treatment. Due to the prolonged half-life, significant levels may persist for weeks after cessation of therapy.
• Mitotane is lipophilic and accumulates in adipose tissue. Despite maintaining a constant dose, mitotane levels may suddenly increase. Monitor mitotane plasma levels even when Mitotane has been withheld as adipose tissue may continue to release mitotane. Due to adipose tissue accumulation of mitotane, close monitoring of mitotane plasma levels is recommended in overweight patients and patients with recent weight loss.
• Swallow Mitotane tablets whole; do not crush, chew or split.
• Take Mitotane with food. Timing of the dose relative to meals must be consistent. Administration with high fat food enhances absorption.
• Do not take tablets showing signs of deterioration.
• Caregivers should wear disposable gloves when handling the tablets. Avoid exposure to crushed and/or broken tablets. If contact with crushed/broken tablets occurs, wash the contaminated skin immediately and thoroughly.
• If a patient misses a dose, instruct the patient to take the next dose as scheduled.
• If a patient vomits after taking a dose, instruct the patient to take the next dose as scheduled.
The recommended dosage reduction for adverse reactions is to decrease the usual daily dose by 500-1000 mg.
Table 1 describes the dosage modifications for specific adverse reactions.
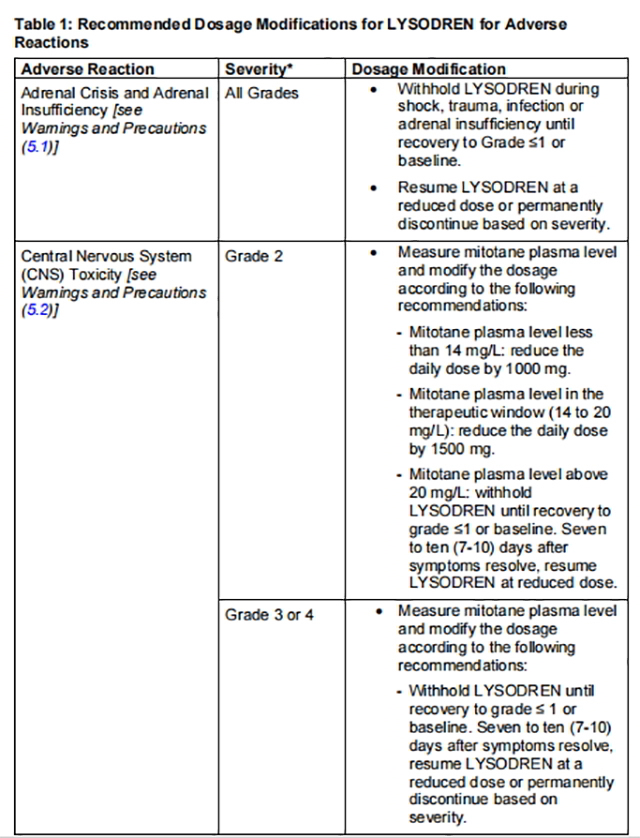
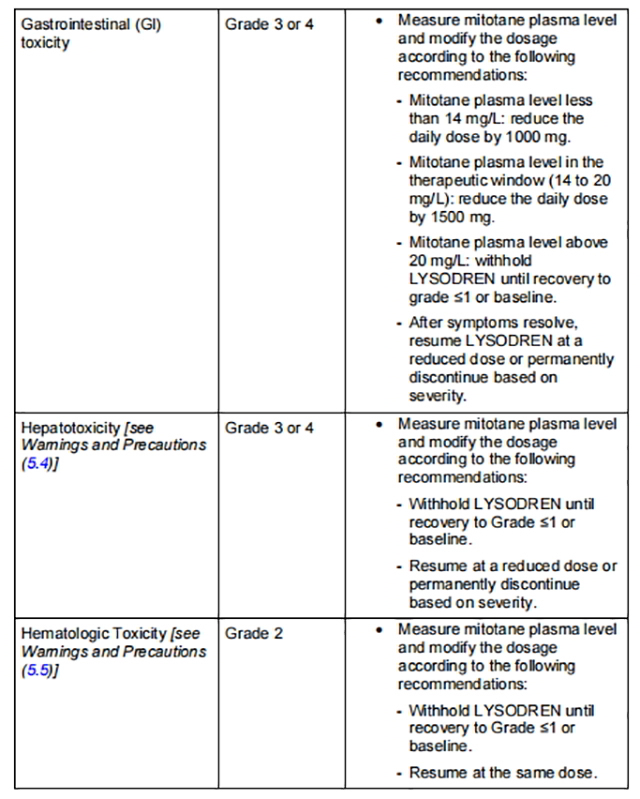
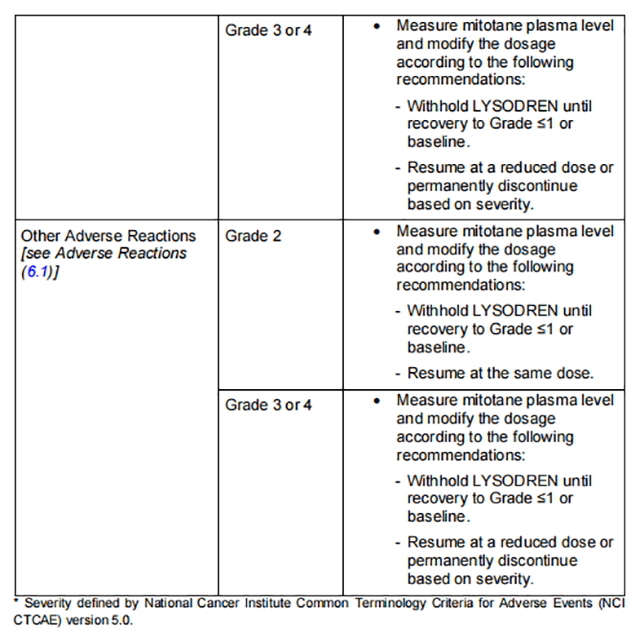
from FDA,2024.01
Mitotan has important value in the treatment of specific diseases.How to use mit···【more】
Release date:2025-04-30Recommended:167
Standardized drug use and rational management of the treatment process play a ke···【more】
Release date:2025-04-29Recommended:131
Mastering the drug dosage specification is an important prerequisite for improvi···【more】
Release date:2025-04-29Recommended:131
Mitotanecan inhibit the function of adrenal cortical cells, especially the activ···【more】
Release date:2024-08-19Recommended:204
Mitotaneworks primarily by inhibiting the function of adrenal cortical cells. It···【more】
Release date:2024-08-19Recommended:301
Mitotaneis a medication used to treat specific types of cancer and adrenal gland···【more】
Release date:2024-08-19Recommended:312