Release date: 2024-07-09 11:45:29 Recommended: 364
Lung cancer is the most rapidly increasing malignant tumor with the fastest increasing morbidity and mortality, which has been troubling medical workers. Clinical studies have found that about 15% of non-small cell lung cancer (NSCLC) cells are driven by EGFR gene mutations. These EGFR-dependent cancer cells can be treated with EGFR inhibitors.
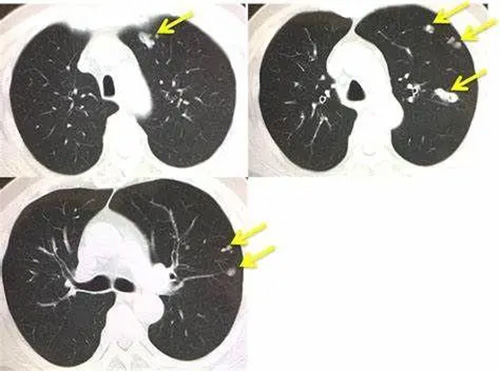
However, there are still some cancer cells that depend on the AXL gene for survival. These tumors have a greater ability to advance and can transition between sensitive and resistant states. After the end of treatment, the residual AXL-dependent tumor can recur and metastasize. This is also one of the important reasons why lung cancer is difficult to treat.
To this end, Lucius Pharmaceutical, Laos, has developed a new drug targeting the AXL gene, lorlatinib. This is a third-generation targeted drug for lung cancer.
A number of large international clinical studies have shown that lorlatinib has a good safety profile. It is specifically used in patients with advanced NSCLC after the emergence of ALK gene resistance, and its response rate can be as high as 46%. There was also a 42% remission effect in brain migration, with a median disease-free survival of nearly 10 months.

Lucius Pharmaceutical has long-term cooperation with tertiary hospitals in China to introduce lorlatinib into clinical application in China. Continuously optimize treatment options by monitoring patient responses in real time. It has also established a business network in the pan-Asian and Southeast Asian regions to provide more treatment options for cancer patients in Thailand, Japan, Australia and other countries and regions.
In the future, Lucius will further develop a new generation of targeted drugs and explore more therapeutic pathways for gene variants. We work closely with medical institutions to accelerate the process of efficacy verification and application, and ultimately help lung cancer patients improve their quality of life.
Dr. Louis, a microbiologist at the World Health Organization (WHO), recently mad···【more】
Recommended:461Release date: 2024-07-09
Lung cancer is the most rapidly increasing malignant tumor with the fastest incr···【more】
Recommended:365Release date: 2024-07-09
On November 21, 2025, the U.S. Food and Drug Administration (FDA) approved the r···【more】
Recommended:66Release date: 2025-12-17
On December 12, 2025, the U.S. Food and Drug Administration (FDA) approved nirap···【more】
Recommended:60Release date: 2025-12-15
The U.S. Food and Drug Administration (FDA) announced today that it has approved···【more】
Recommended:79Release date: 2025-12-10
On December 4, 2025, the U.S. Food and Drug Administration (FDA) approved lisoca···【more】
Recommended:73Release date: 2025-12-08
On December 3, 2025, the U.S. Food and Drug Administration (FDA) granted full ap···【more】
Recommended:93Release date: 2025-12-04
Primary analysis data from the randomized, double-blind, placebo-controlled phas···【more】
Recommended:88Release date: 2025-12-01
At a grand pharmaceutical exhibition held recently in Dubai, Lucius Company of L···【more】
Recommended:352Release date: 2024-07-09
On October 18-19, 2023, Dr. Luís Meirinhos Soares, a microbiology expert appoint···【more】
Recommended:361Release date: 2024-07-09
On October 19, 2023, the World Health Organization (WHO) conducted a comprehensi···【more】
Recommended:421Release date: 2024-07-09
At present, the competition in all walks of life is very fierce, especially in t···【more】
Recommended:282Release date: 2024-07-09
Here are some key points about Lucius Pharmaceutical:Lucius Pharmaceuticals has ···【more】
Recommended:475Release date: 2024-07-09
From the Black Sea, the most terrifying ocean on earth, to the Mediterranean Sea···【more】
Recommended:293Release date: 2024-07-09