The efficacy of Quizartinib in combination with chemotherapy was evaluated in QuANTUM-First (NCT02668653), a randomized, double-blind, placebo-controlled study of 539 patients with newly diagnosed FLT3-ITD positive AML.
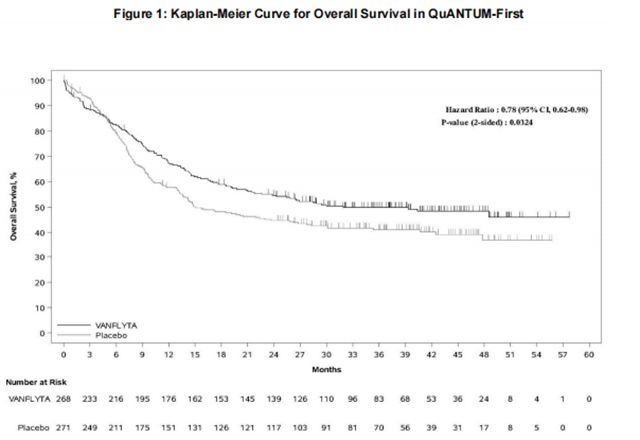
Efficacy was established on the basis of overall survival (OS), measured from the date of randomization until death by any cause. The primary analysis was conducted after a minimum follow-up of 24 months after the randomization of the last patient. The study demonstrated a statistically significant improvement in OS for the Quizartinib arm [hazard ratio (HR) 0.78; 95% CI: 0.62, 0.98; 2-sided p=0.0324] (see Figure 1).
In an exploratory subgroup analysis of the 89/208 (43%) of patients who received maintenance therapy with Quizartinib or placebo following consolidation chemotherapy, the OS HR was 0.40 (95% CI: 0.19, 0.84). Of 119/208 (57%) of patients who received maintenance therapy with Quizartinib or placebo following HSCT, the OS HR was 1.62 (95% CI: 0.62, 4.22).
The CR rate in the Quizartinib arm was 55% (95% CI: 48.7, 60.9) with a median duration of CR of 38.6 months (95% CI: 21.9, NE), and the CR rate in the placebo arm was 55% (95% CI: 49.2, 61.4) with a median duration of CR of 12.4 months (95% CI: 8.8, 22.7).
from FDA,2023.07