Select patients for the treatment of AML with Quizartinib based on the presence of FLT3-ITD mutation positivity.
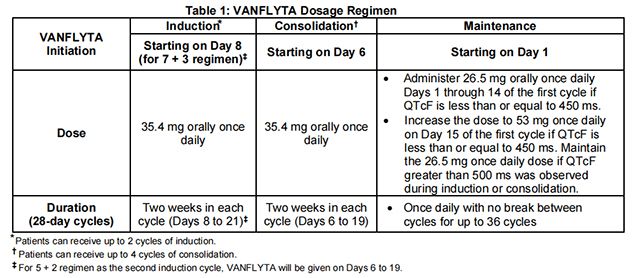
A treatment course consists of up to 2 cycles of Quizartinib in combination with induction cytarabine and anthracycline, up to 4 cycles of Quizartinib in combination with high-dose cytarabine consolidation, and up to 36 cycles of Quizartinib as maintenance therapy or until disease progression or unacceptable toxicity. Quizartinib maintenance therapy should be initiated following consolidation chemotherapy upon blood count recovery of absolute neutrophil count >500/mm3 and platelet count >50,000/mm3.
See Table 1 for the recommended dosage of Quizartinib by phase of therapy.
For patients who proceed to hematopoietic stem cell transplantation (HSCT), Quizartinib should be stopped 7 days before the start of a conditioning regimen.
Administer Quizartinib orally with or without food at approximately the same time each day. Swallow tablets whole. Do not cut, crush, or chew the tablets. If a dose of Quizartinib is vomited, do not administer a replacement dose; wait until the next scheduled dose is due. If a dose of Quizartinib is missed or not taken at the usual time, administer the dose as soon as possible on the same day and return to the usual schedule the following day. The patient should not take two doses on the same day.
Initiate Quizartinib only if QTcF is less than or equal to 450 ms.
During induction and consolidation, perform ECGs prior to initiation and then once weekly during Quizartinib treatment or more frequently as clinically indicated.
During maintenance, perform ECGs prior to initiation, once weekly for at least the first month following dose initiation and escalation, and thereafter as clinically indicated. Escalate the dose only if QTcF is less than or equal to 450 ms.
Correct electrolyte abnormalities (hypokalemia and hypomagnesemia), and if possible, avoid concomitant administration of drugs that prolong the QT interval.
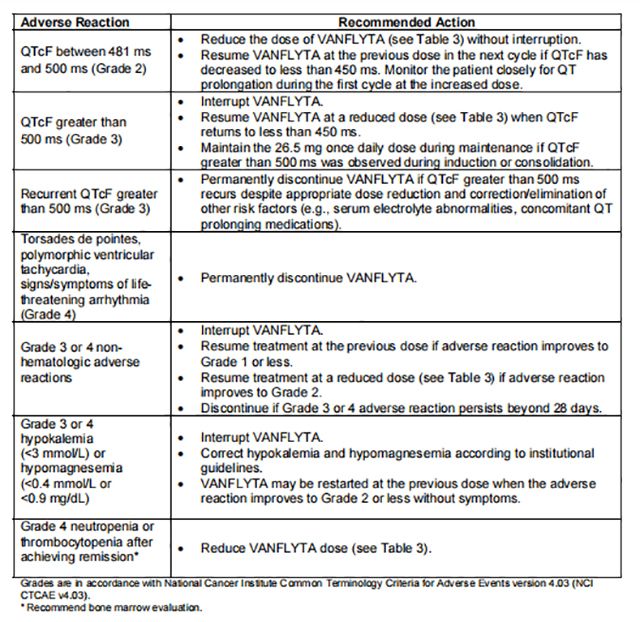
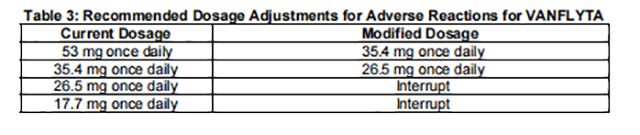
For recommended dosage modifications due to adverse reactions, see Table 2. For dosage adjustments due to adverse reactions, see Table 3.

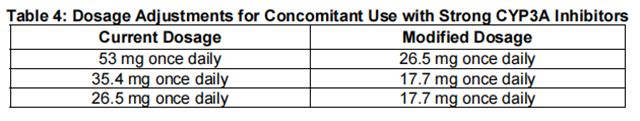
Reduce the dosage of Quizartinib when used concomitantly with strong CYP3A inhibitors as shown in Table 4. If the current dosage is 17.7 mg once daily, interrupt Quizartinib treatment for the duration of strong CYP3A inhibitor use. After discontinuation of a strong CYP3A inhibitor for 5 half-lives, resume the Quizartinib dose that was taken before initiating the strong inhibitor.
from FDA,2023.07