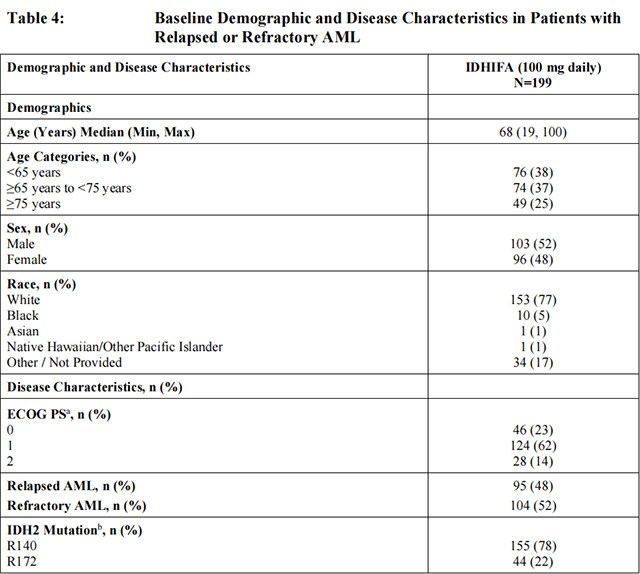
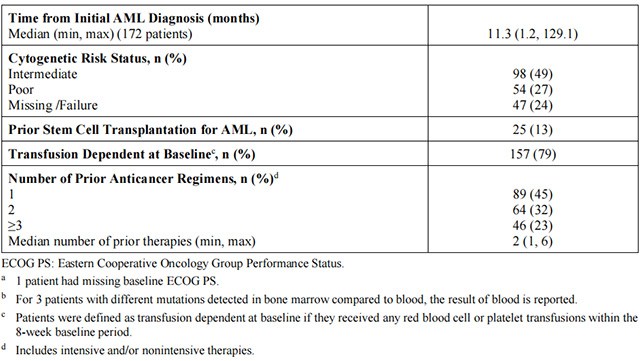
The efficacy of Enasidenib was evaluated in an open-label, single-arm, multicenter, two-cohort clinical trial (Study AG221-C-001, NCT01915498) of 199 adult patients with relapsed or refractory AML and an IDH2 mutation, who were assigned to receive 100 mg daily dose. Cohort 1 included 101 patients and Cohort 2 included 98 patients. IDH2 mutations were identified by a local diagnostic test and retrospectively confirmed by the Abbott RealTime IDH2 assay, or prospectively identified by the Abbott RealTime IDH2 assay, which is the FDA-approved test for selection of patients with AML for treatment with Enasidenib. Enasidenib was given orally at starting dose of 100 mg daily until disease progression or unacceptable toxicity. Dose reductions were allowed to manage adverse events.
The baseline demographic and disease characteristics are shown in Table 4. The baseline demographics and disease characteristics were similar in both study cohort.
FDA,2023.12
With the continuous progress of medicine, the application of the new drug encidi···【more】
Release date:2024-12-27Recommended:209
With the continuous deepening of medical research, the new drug encidipine has e···【more】
Release date:2024-12-27Recommended:221
Encidipine is a new type of drug that has shown significant results in the treat···【more】
Release date:2024-12-27Recommended:273