Select patients for the treatment of metastatic NSCLC with Crizotinib based on the presence of ALK or ROS1 positivity in tumor specimens.
• Monitor liver function tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin, every 2 weeks during the first 2 months of treatment, then once a month, and as clinically indicated, with more frequent repeat testing for increased liver transaminases, alkaline phosphatase, or total bilirubin in patients who develop increased transaminases.
• Monitor complete blood counts including differential weekly for the first month of therapy and then at least monthly, with more frequent monitoring if Grade 3 or 4 abnormalities, fever, or infection occur.
• For pediatric and young adult patients with ALCL or pediatric patients with IMT, obtain baseline and follow-up ophthalmologic examinations including retinal examination within 1 month of starting Crizotinib and every 3 months thereafter.
The recommended dosage of Crizotinib is provided in Table 1.
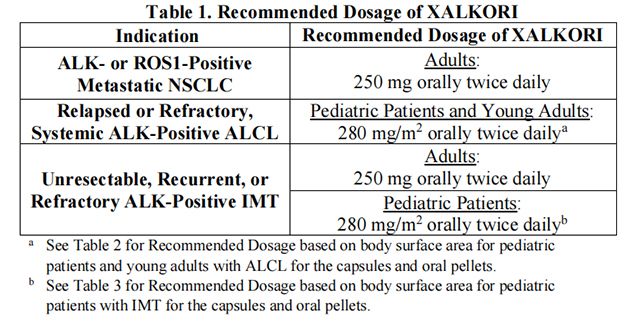
• The recommended dosage for adult patients with ALK- or ROS1-positive metastatic NSCLC is Crizotinib capsules 250 mg orally, twice daily, with or without food until disease progression or unacceptable toxicity occurs.
• For adults who cannot swallow capsules, the recommended dosage of Crizotinib pellets is 250 mg (2 x 50 mg + 1 x 150 mg) orally, twice daily, with or without food until disease progression or unacceptable toxicity occurs.
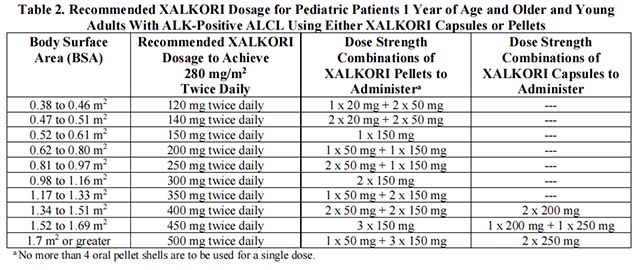
• The recommended dosage for pediatric patients 1 year of age and older and young adults with relapsed or refractory, systemic ALK-positive ALCL is based on body surface area (BSA) and is provided in Table 2.
• Administer Crizotinib capsules or pellets orally, twice daily, with or without food until disease progression or unacceptable toxicity occurs.
Table 2 provides the dosage based on body surface area (BSA) for Crizotinib capsules or pellets.
• The recommended dosage for adult patients with unresectable, recurrent, or refractory ALK-positive IMT is provided in Table 1.
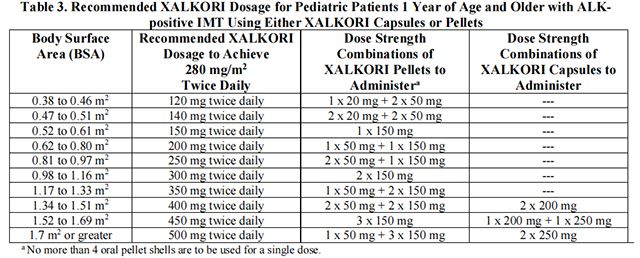
• The recommended dosage for pediatric patients 1 year of age and older with unresectable, recurrent, or refractory ALK-positive IMT is based on BSA and is provided in Table 3.
• Administer Crizotinib capsules or pellets orally twice daily, with or without food, until disease progression or unacceptable toxicity occurs.
Table 3 provides the dosage based on BSA for Crizotinib capsules or pellets.
• Administer Crizotinib capsules or pellets orally, twice daily, with or without food.
• If a dose of Crizotinib capsules or pellets is missed, make up that dose unless the next dose is due within 6 hours.
• If vomiting occurs after taking a dose of Crizotinib capsules or pellets, do not take an additional dose. Take the next dose at the regular scheduled time.
• Swallow Crizotinib capsules whole, with or without food twice daily.
• Do not chew, crush or split Crizotinib capsules.
• Crizotinib pellets are supplied encapsulated in shells.
• Do not chew or crush Crizotinib pellets.
• Do not swallow Crizotinib pellets encapsulated in the shell.
• Crizotinib pellets can be administered by 2 options:
1. Open shell(s) containing Crizotinib pellets and empty the contents directly into the patient’s mouth.
2. Open shell(s) containing Crizotinib pellets and empty the contents into a consumer -supplied oral dosing aid (e.g., spoon, medicine cup). Administer Crizotinib pellets via the dosing aid directly into the patient’s mouth.
• Immediately after administration, give a sufficient amount of water to ensure that all medication is swallowed.
Antiemetics are recommended prior to and during treatment with Crizotinib to prevent nausea and vomiting. Provide standard antiemetic and antidiarrheal agents for gastrointestinal toxicities.
Consider intravenous or oral hydration for patients at risk of dehydration, and replace electrolytes as clinically indicated.
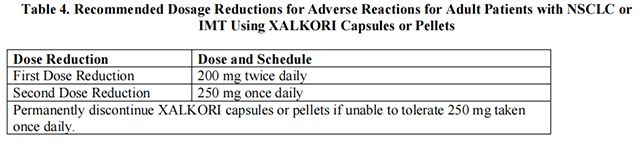
The recommended dosage modifications for adverse reactions for adult patients with NSCLC or IMT are provided in Table 4.
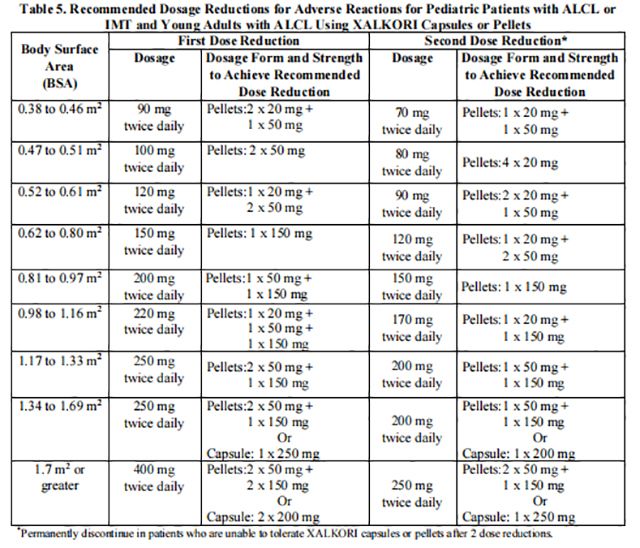
The recommended dosage modifications for adverse reactions for pediatric patients with ALCL or IMT and young adults with ALCL are based on body surface area and are provided in Table 5.
The recommended dosage modifications for hematologic adverse reactions for adult patients with NSCLC or IMT are provided in Table 6.

Monitor complete blood counts including differential weekly for the first month of therapy and then at least monthly, with more frequent monitoring if Grade 3 or 4 abnormalities, fever, or infection occur.
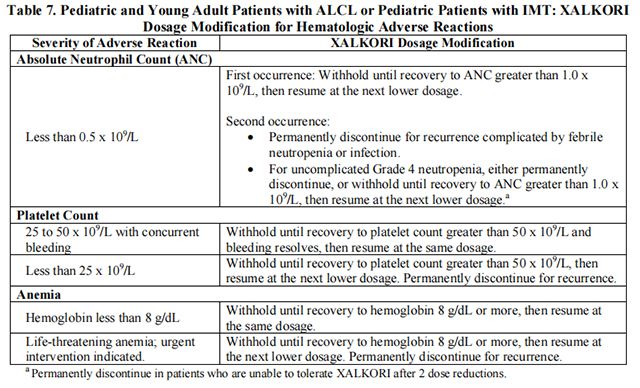
The recommended dosage modifications for hematologic adverse reactions in pediatric and young adult patients with ALCL or pediatric patients with IMT are provided in Table 7.
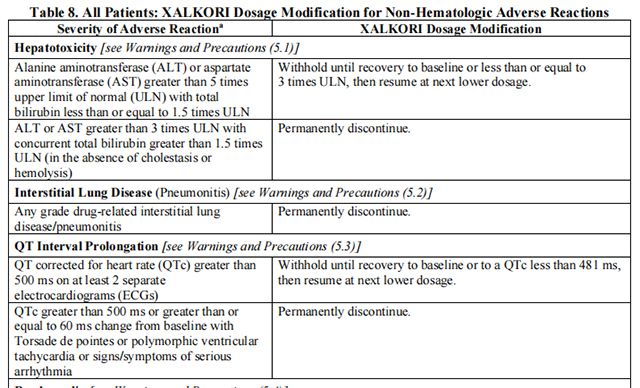
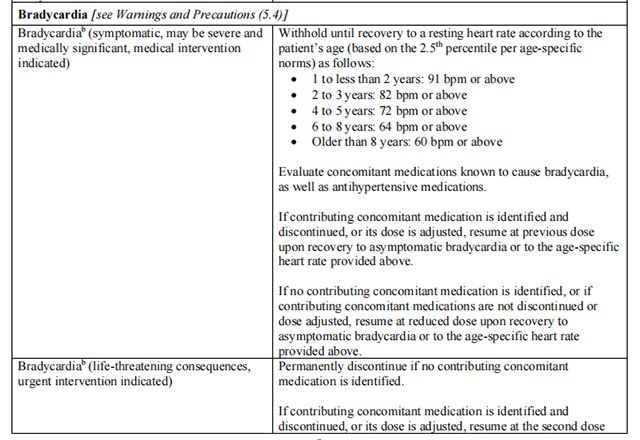
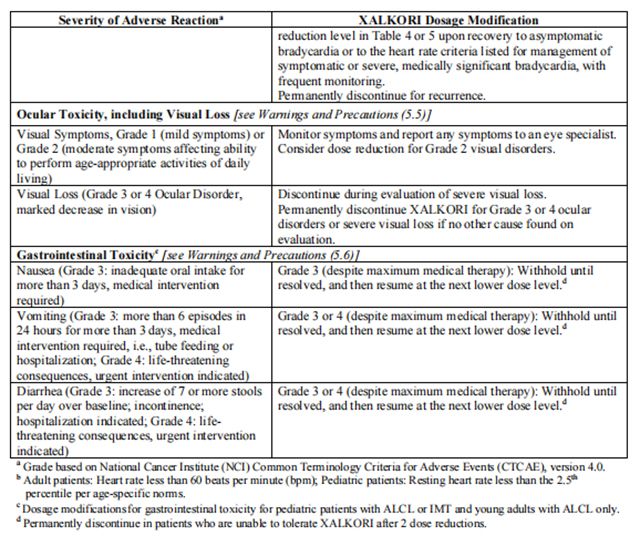
The recommended dosage modifications for non-hematologic adverse reactions are provided in Table 8.
The recommended dose of Crizotinib in patients with moderate hepatic impairment [any aspartate aminotransferase (AST) and total bilirubin greater than 1.5 times the upper limit of normal (ULN) and less than or equal to 3 times ULN] is the first dose reduction shown in Table 4 for adult patients with NSCLC or IMT and Table 5 for pediatric patients with ALCL or IMT and young adults with ALCL.
The recommended dose of Crizotinib in patients with severe hepatic impairment (any AST and total bilirubin greater than 3 times ULN) is the second dose reduction shown in Table 4 for adult patients with NSCLC or IMT and Table 5 for pediatric patients with ALCL or IMT and young adults with ALCL.
The recommended dosage of Crizotinib in patients with severe renal impairment [creatinine clearance (CLcr) less than 30 mL/min, calculated using the modified Cockcroft-Gault equation for adult patients and the Schwartz equation for pediatric patients] not requiring dialysis is the second dose reduction shown in Table 4 for adult patients with NSCLC or IMT and Table 5 for pediatric patients with ALCL or IMT and young adults with ALCL.
Avoid concomitant use of strong CYP3A inhibitors. If concomitant use of strong CYP3A inhibitors is unavoidable, reduce the dose of Crizotinib to the second dose reduction shown in Table 4 for adult patients with NSCLC or IMT and Table 5 for pediatric patients with ALCL or IMT and young adults with ALCL. After discontinuation of a strong CYP3A inhibitor, resume the Crizotinib dose used prior to initiating the strong CYP3A inhibitor.
from FDA,2023.09
Targeted therapy options for patients with specific gene mutations have sparked ···【more】
Release date:2025-04-02Recommended:194
This article discusses the standardized use and precautions of the drug crizotin···【more】
Release date:2025-04-02Recommended:170
The following article will introduce in detail the standardized medication guida···【more】
Release date:2025-04-01Recommended:164
When it comes to drug use norms and cost planning, scientific understanding and ···【more】
Release date:2025-04-01Recommended:183