Perform the following tests and evaluations prior to Cibinqo initiation:
Tuberculosis (TB) infection evaluation – Cibinqo initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of Cibinqo.
Viral hepatitis screening in accordance with clinical guidelines-Cibinqo initiation is not recommended in patients with active hepatitis B or hepatitis C.
A complete blood count (CBC)- Cibinqo initiation is not recommended in patients with a platelet count <150,000/mm3 , an absolute lymphocyte count <500/mm3 , an absolute neutrophil count <1,000/mm3 , or a hemoglobin value<8 g/dL.
Complete any necessary immunizations, including herpes zoster vaccinations, in agreement with current immunization guidelines prior to Cibinqo initiation.
The recommended dose is 100 mg once daily.
If an adequate response is not achieved with Cibinqo 100 mg once daily, consider increasing the dosage to 200 mg once daily. Discontinue Cibinqo if an adequate response is not achieved with 200 mg once daily.
Use the lowest efficacious dose to maintain response.
Cibinqo can be used with or without topical corticosteroids.
If a dose is missed, administer the dose as soon as possible unless it is less than 12 hours before the next dose, in which case skip the missed dose. Thereafter, resume dosing at the regular scheduled time.
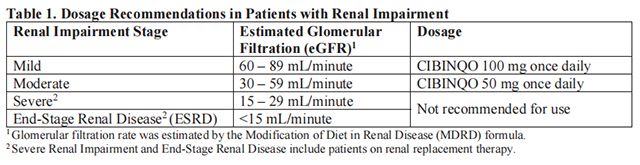
Cibinqo dosage recommendations for patients with renal impairment are provided in Table 1. In patients with mild and moderate renal impairment, if an adequate response is not achieved with initial dose, the dose of Cibinqo can be doubled.
Cibinqo is not recommended for use in patients with severe hepatic impairment.
In patients who are known or suspected to be CYP2C19 poor metabolizers, the recommended dosage of Cibinqo is 50 mg once daily. If an adequate response is not achieved with Cibinqo 50 mg once daily, consider increasing the dosage to 100 mg once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
In patients taking strong inhibitors of cytochrome P450 (CYP) 2C19, reduce the dosage to 50 mg once daily. If an adequate response is not achieved with Cibinqo 50 mg daily, consider increasing the dosage to 100 mg once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
If a patient develops a serious or opportunistic infection, discontinue Cibinqo and control the infection. The risks and benefits of treatment with Cibinqo should be carefully considered prior to reinitiating therapy with Cibinqo.
Recommendations for Cibinqo discontinuation for laboratory abnormalities are summarized in Table 2.

CBC evaluations are recommended at baseline, 4 weeks after treatment initiation and 4 weeks after dosage increase of Cibinqo. Laboratory evaluations may be extended for patients on chronic Cibinqo therapy who develop hematologic abnormalities.
Administer Cibinqo with or without food at approximately the same time each day. Swallow Cibinqo tablets whole with water. Do not crush, split, or chew Cibinqo tablets.
from FDA,2023.12
Knowing the correct dosage of abuxitinib is critical to improving the effectiven···【more】
Release date:2025-06-06Recommended:125
Understanding the correct way to use abuxitinib is critical to the effectiveness···【more】
Release date:2025-06-06Recommended:131
Understanding the dosage of the drug is critical to the course of treatment.What···【more】
Release date:2025-05-19Recommended:164