The efficacy of Avatrombopag for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo a procedure was established in 2 identically-designed multicenter, randomized, double-blind, placebo-controlled trials (ADAPT-1 [NCT01972529] and ADAPT-2 [NCT01976104]). In each trial, patients were assigned to the Low Baseline Platelet Count Cohort (<40×109/L) or the High Baseline Platelet Count Cohort (≥40 to <50×109/L) based on their platelet count at baseline. Patients were then randomized in a 2:1 ratio to either Avatrombopag or placebo. Patients were stratified according to hepatocellular cancer (HCC) status and risk of bleeding associated with the elective procedure (low, moderate, or high). Patients undergoing neurosurgical interventions, thoracotomy, laparotomy or organ resection were not eligible for enrollment.
Patients in the Low Baseline Platelet Count Cohort received 60 mg Avatrombopag or matching placebo once daily for 5 days, and patients in the High Baseline Platelet Count Cohort received 40 mg Avatrombopag or matching placebo once daily for 5 days. Eligible patients were scheduled to undergo their procedure (low, moderate, or high bleeding risk) 5 to 8 days after their last dose of treatment. Patient populations were similar between the pooled Low and High Baseline Platelet Count Cohorts and consisted of 66% male and 35% female; median age 58 years and 61% White, 34% Asian, and 3% Black.
Responders were defined as patients who did not require a platelet transfusion or any rescue procedure for bleeding after randomization and up to 7 days following a scheduled procedure. The following were considered rescue therapies to manage the risk of bleeding associated with a procedure: whole blood transfusion, packed red blood cell (RBC) transfusion, platelet transfusion, fresh frozen plasma (FFP) or cryoprecipitate administration, Vitamin K, desmopressin, recombinant activated factor VII, aminocaproic acid, tranexamic acid, or surgical or interventional radiology procedures performed to achieve hemostasis and control blood loss. In both baseline platelet count cohorts, patients in the Avatrombopag treatment groups had a greater proportion of responders than the corresponding placebo treatment groups that was both clinically meaningful and statistically significant as detailed in Table 8.
Table 8: Proportion of Patients Not Requiring a Platelet Transfusion or Any Rescue Procedure for Bleeding by Baseline Platelet Count Cohort and Treatment Group – ADAPT-1 and ADAPT-2
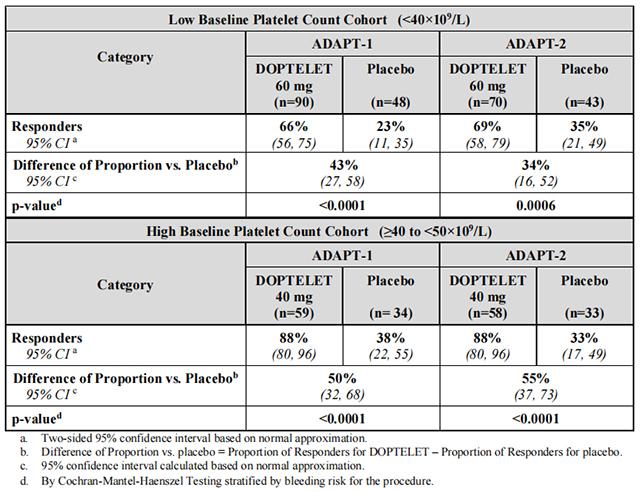
In addition, both trials demonstrated a higher proportion of patients who achieved the target platelet count of ≥50×109 /L on the day of procedure, a secondary efficacy endpoint, in both Avatrombopag-treated groups versus the placebo-treated groups for both cohorts (Low Baseline Platelet Count Cohort – ADAPT-1: 69% vs 4%, respectively; p<0.0001, ADAPT-2: 67% vs 7%, respectively; p <0.0001; High Baseline Platelet Count Cohort – ADAPT-1: 88% vs 21%, respectively; p <0.0001: ADAPT-2: 93% vs 39%, respectively; p <0.0001). Further, both trials demonstrated a greater mean change in platelet counts from baseline to the day of the procedure, a secondary efficacy endpoint, in both Avatrombopag-treated groups versus the placebo-treated groups for both cohorts (Low Baseline Platelet Count Cohort – ADAPT-1: 32×109 /L vs 0.8×109 /L, respectively; p<0.0001; ADAPT-2: 31.3×109 /L vs 3.0×109 /L, respectively; p <0.0001; High Baseline Platelet Count Cohort – ADAPT 1: 37.1×109 /L vs 1.0×109 /L, respectively; p <0.0001; ADAPT-2: 44.9×109 /L vs 5.9×109 /L, respectively; p <0.0001).
A measured increase in platelet counts was observed in both Avatrombopag treatment groups over time beginning on Day 4 post-dose, that peaked on Day 10-13, decreased 7 days post-procedure, and then returned to near baseline values by Day 35.
The efficacy of Avatrombopag in adult patients with chronic immune thrombocytopenia was evaluated in a Phase 3, multicenter, randomized, double-blind, placebo-controlled trial (NCT01438840). Patients had previously received one or more prior chronic immune thrombocytopenia therapies and had an average of screening and baseline platelet counts <30×109 /L. Patients were centrally stratified by splenectomy status, baseline platelet count (≤15×109 /L or >15×109 /L to <30×109 /L), and use of concomitant chronic immune thrombocytopenia medication, and then randomized (2:1) to receive either Avatrombopag or placebo for 6 months. Patients received a starting dose of 20 mg once daily, with doses subsequently titrated based on platelet response.
Forty-nine patients were randomized, 32 to Avatrombopag and 17 to placebo, with similar mean [SD] baseline platelet counts in the 2 treatment groups (14.1 [8.6]×109 /L and 12.7 [7.8]×109 /L, respectively). The median age was 44 years, 63% were female, and 94% were Caucasian, 4% Asian and 2% Black. The median duration of exposure was 26 weeks for Avatrombopag-treated patients and 6 weeks for placebo-treated patients. The major efficacy outcome in this trial was the cumulative number of weeks in which the platelet count was ≥50×109 /L during the 6-month treatment period in the absence of rescue therapy. Avatrombopag-treated patients had a longer duration of platelet counts ≥50×109 /L in the absence of rescue therapy than those who received placebo (median 12.4 [0, 25] vs 0 [0, 2] weeks, respectively, p <0.0001) (see Table 9).
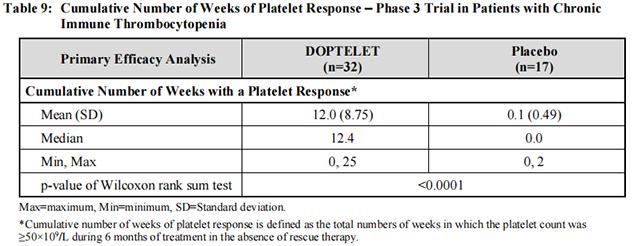
In addition, a larger proportion of patients in the Avatrombopag treatment group had platelet counts ≥50×109 /L at Day 8 compared to placebo (21/32; 66% vs 0/17; 0.0%, respectively; p <0.0001).
from FDA,2021.06
Avatrombopag is a novel thrombopoietin receptor agonist, and its therapeutic eff···【more】
Release date:2025-01-22Recommended:219
Avatrombopag is a novel thrombopoietin receptor agonist that has attracted much ···【more】
Release date:2025-01-22Recommended:218
Avatrombopag is a new oral thrombopoietin receptor agonist, which has been widel···【more】
Release date:2025-01-21Recommended:217