Begin Avatrombopag dosing 10 to 13 days prior to the scheduled procedure. The recommended daily dose of Avatrombopag is based on the patient’s platelet count prior to the scheduled procedure (see Table 1). Patients should undergo their procedure 5 to 8 days after the last dose of Avatrombopag.
Avatrombopag should be taken orally once daily for 5 consecutive days with food. In the case of a missed dose, patients should take the next dose of Avatrombopag as soon as they remember. Patients should not take two doses at one time to make up for a missed dose, and should take the next dose at the usual time the next day; all 5 days of dosing should be completed.
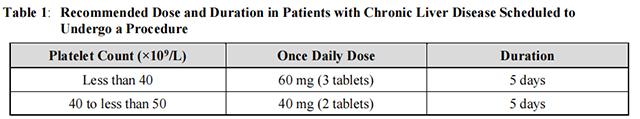
Avatrombopag has been investigated only as a single 5-day once daily dosing regimen in clinical trials in patients with chronic liver disease. Avatrombopag should not be administered to patients with chronic liver disease in an attempt to normalize platelet counts.
Use the lowest dose of Avatrombopag needed to achieve and maintain a platelet count greater than or equal to 50×109 /L as necessary to reduce the risk for bleeding. Dose adjustments are based on platelet count response. Do not use Avatrombopag to normalize platelet counts.
Begin Avatrombopag at a starting dose of 20 mg (1 tablet) once daily with food.
After initiating therapy with Avatrombopag, assess platelet counts weekly until a stable platelet count greater than or equal to 50×109 /L has been achieved, and then obtain platelet counts monthly thereafter. Obtain platelet counts weekly for at least 4 weeks following discontinuation of Avatrombopag.
Dose adjustments (see Table 2 and Table 3) are based on the platelet count response. Do not exceed a daily dose of 40 mg (2 tablets).
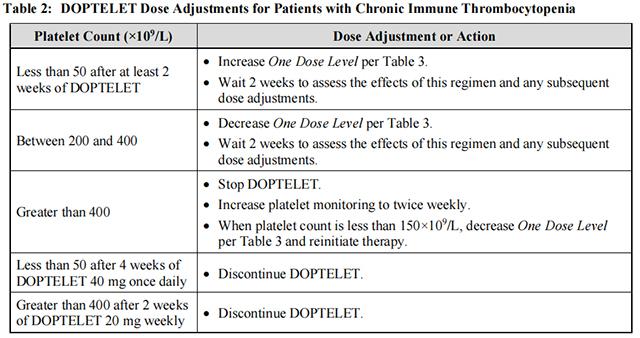
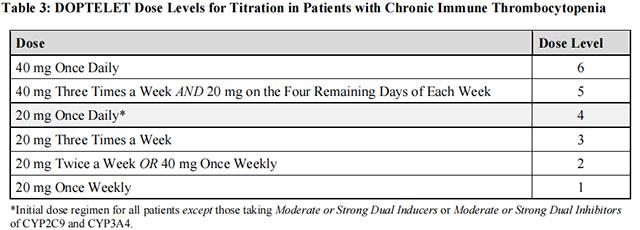
In the case of a missed dose, patients should take the missed dose of Avatrombopag as soon as they remember. Patients should not take two doses at one time to make up for a missed dose, and should take the next dose per the current regimen.
Discontinue Avatrombopag if the platelet count does not increase to greater than or equal to 50×109 /L after 4 weeks of dosing at the maximum dose of 40 mg once daily. Discontinue Avatrombopag if the platelet count is greater than 400×109 /L after 2 weeks of dosing at 20 mg once weekly.
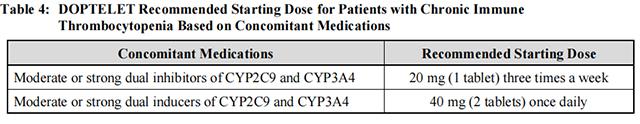
The recommended starting doses of Avatrombopag in patients with chronic immune thrombocytopenia receiving concomitant medications are summarized in Table 4.
from FDA,2021.06
Avatrombopag is a novel thrombopoietin receptor agonist, which has attracted muc···【more】
Release date:2025-01-21Recommended:171
Avatrombopag is a new type of drug, and its correct use is critical to the patie···【more】
Release date:2025-01-20Recommended:133
Avatrombopag is a novel oral thrombopoietin receptor agonist, and its dosage has···【more】
Release date:2025-01-20Recommended:216