The efficacy of Asciminib in the treatment of patients with Ph+ CML in chronic phase (Ph+ CML-CP), previously treated with two or more tyrosine kinase inhibitors was evaluated in the multi-center, randomized, active-controlled, and open-label study Asciminib (NCT 03106779).
In this study, a total of 233 patients were randomized in a 2:1 ratio and stratified according to major cytogenetic response (MCyR) status to receive either Asciminib 40 mg twice daily (N = 157) or bosutinib 500 mg once daily (N = 76). Patients continued treatment until unacceptable toxicity or treatment failure occurred.
Patients were 52% female and 48% male with a median age of 52 years (range, 19 to 83 years). Of the 233 patients, 19% were 65 years or older, while 2.6% were 75 years or older. Patients were White (75%), Asian (14%), and Black or African American (4.3%). Of the 233 patients, 81% and 18% had Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, respectively. Patients who had previously received 2, 3, 4, or 5 or more prior lines of TKIs were 48%, 31%, 15%, and 6%, respectively. The median duration of treatment was 67 weeks (range, 0.1 to 162 weeks) for patients receiving Asciminib and 30 weeks (range, 1 to 149 weeks) for patients receiving bosutinib.
The main efficacy outcomes from Asciminib are summarized in Table 8.
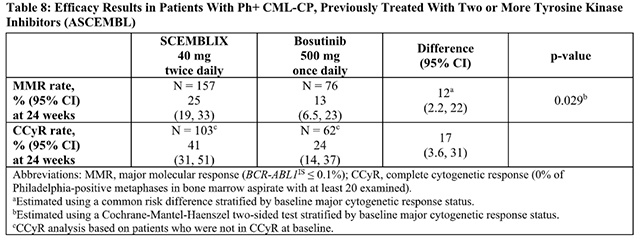
The MMR rate at 48 weeks was 29% (95% CI: 22, 37) in patients receiving Asciminib and 13% (95% CI: 6.5, 23) in patients receiving bosutinib. With a median duration of follow-up of 20 months (range: 1 day to 36 months), the median duration of response had not yet been reached for patients with MMR at any time.
The efficacy of Asciminib in the treatment of patients with Ph+ CML-CP with the T315I mutation was evaluated in a multi-center open-label study CABL001X2101 (NCT02081378). Testing for T315I mutation utilized a qualitative p210 BCR-ABL mutation test using Sanger Sequencing.
Efficacy was based on 45 patients with Ph+ CML-CP with the T315I mutation who received Asciminib at a dose of 200mg twice daily. Patients continued treatment until unacceptable toxicity or treatment failure occurred.
Of the 45 patients, 80% were male and 20% female; 31% were 65 years or older, while 9% were 75 years or older with a median age of 54 years (range, 26 to 86 years). The patients were White (47%), Asian (27%), and Black or African American (2.2%), and 24% were unreported or unknown. Seventy-three percent and 27% of patients had ECOG performance status 0 and 1, respectively. Patients who had previously received 1, 2, 3, 4, and 5 or more TKIs were 18%, 31%, 36%, 13%, and 2.2%, respectively.
MMR was achieved by 24 weeks in 42% (19/45, 95% CI: 28% to 58%) of the 45 patients treated with Asciminib. MMR was achieved by 96 weeks in 49% (22/45, 95% CI: 34% to 64%) of the 45 patients treated with Asciminib. The median duration of treatment was 108 weeks (range, 2 to 215 weeks).
from FDA,2021.10
Advances in the field of cancer treatment are changing the way cancer is treated···【more】
Release date:2024-12-05Recommended:188