There were 341 patients enrolled by tumor tissue in the cohort with a PIK3CA mutation and 231 enrolled in the cohort without a PIK3CA mutation. Of the 341 patients in the cohort with a PIK3CA mutation, 336 (99%) patients had one or more PIK3CA mutations confirmed in tumor tissue using the FDA-approved therascreen® PIK3CA RGQ PCR Kit. Out of the 336 patients with PIK3CA mutations confirmed in tumor tissue, 19 patients had no plasma specimen available for testing with the FDA-approved therascreen® PIK3CA RGQ PCR Kit. Of the remaining 317 patients with PIK3CA mutations confirmed in tumor tissue, 177 patients (56%) had PIK3CA mutations identified in plasma specimen, and 140 patients (44%) did not have PIK3CA mutations identified in plasma specimen.
The major efficacy outcome was investigator-assessed progression-free survival (PFS) in the cohort with a PIK3CA mutation per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Additional efficacy outcome measures were overall response rate (ORR) and overall survival (OS) in the cohort with a PIK3CA mutation.
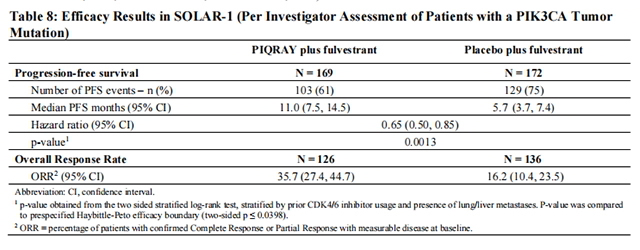
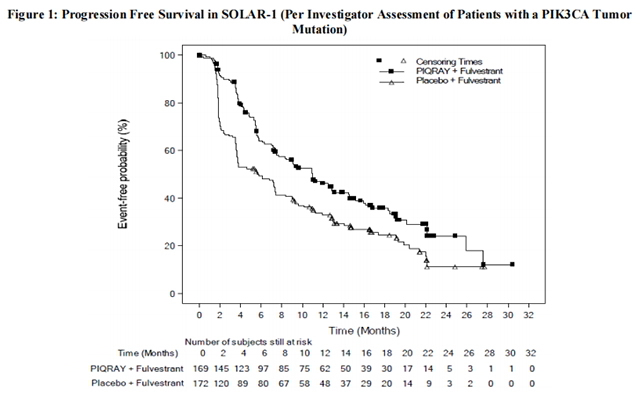
The results from the investigator-assessed PFS and ORR for the cohort with a PIK3CA mutation in tumor tissue are presented in Table 8, and Figure 1. Investigator-assessed PFS results for the cohort with a PIK3CA mutation were supported by consistent results from a blinded independent review committee (BIRC) assessment. Similar results were seen in patients with tissue or plasma PIK3CA mutations. At the pre-specified final OS analysis, there was no significant difference in OS between the PIQRAY plus fulvestrant arm and the placebo plus fulvestrant arm (hazard ratio [HR] = 0.86, 95% CI: 0.64, 1.15).
No benefit was observed in patients whose tumors did not have a PIK3CA tissue mutation (PFS: HR = 0.85, 95% CI: 0.58, 1.25; OS: HR = 0.92, 95% CI: 0.65, 1.29).
from FDA,2022.11
Alpelisib, approved in 2019, has a unique efficacy against PIK3CA mutant breast ···【more】
Release date:2024-08-26Recommended:251
Alpelisib is the first PI3K inhibitor specifically for PIK3CA-mutated breast can···【more】
Release date:2024-08-26Recommended:224
Alpelisib was approved by the FDA in 2019 for the treatment of breast cancer pat···【more】
Release date:2024-08-26Recommended:261