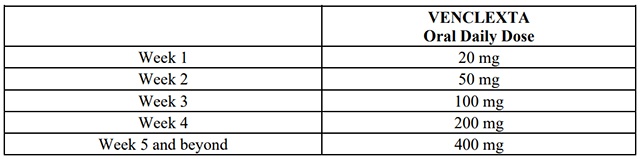
venetoclax dosing begins with a 5-week ramp-up. The 5-week ramp-up dosing schedule is designed to gradually reduce tumor burden (debulk) and decrease the risk of TLS.
venetoclax 5-week Dose Ramp-Up ScheduleAdminister venetoclax according to the 5-week ramp-up dosing schedule to therecommended dosage of 400 mg orally once daily as shown in Table 1.
Table 1. Dosing Schedule for 5-Week Ramp-up Phase for Patients with CLL/SLL
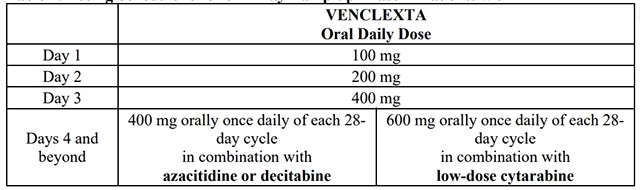
The recommended dosage and ramp-up of venetoclax depends upon the combination agent.Follow the dosing schedule, including the 3-day or 4-day dose ramp-up, as shown in Table 2.Start venetoclax administration on Cycle 1 Day 1 in combination with:
Table 2. Dosing Schedule for 3- or 4-Day Ramp-up Phase in Patients with AML
Patients treated with venetoclax may develop tumor lysis syndrome (TLS). Refer to theappropriate section below for specific details on management. Assess patient-specific factors for level of risk of TLS and provide prophylactic hydration and anti-hyperuricemics to patients priorto first dose of venetoclax to reduce risk of TLS.
Table 3. Recommended TLS Prophylaxis Based on Tumor Burden in Patients with
CLL/SLL
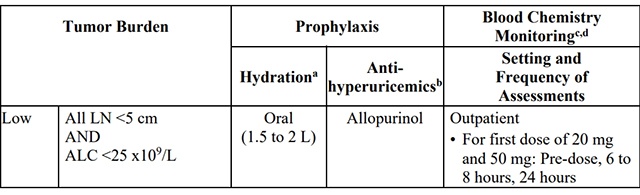
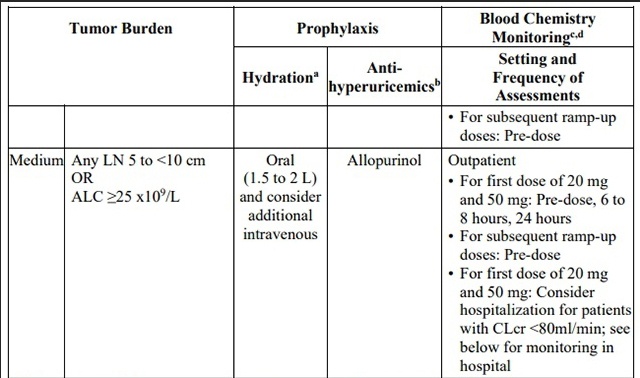
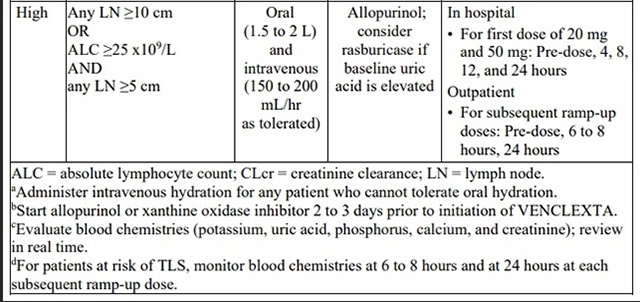
Table 4. Recommended venetoclax Dosage Modifications for Adverse Reactionsa in
CLL/SLL
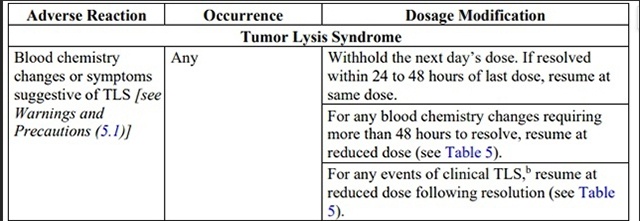
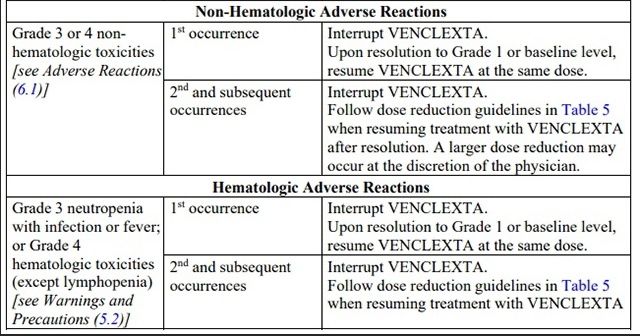
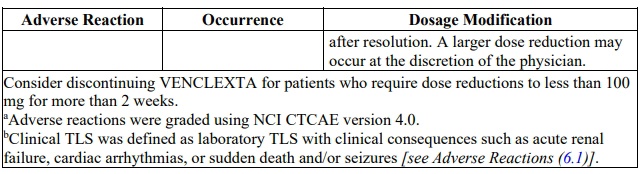
Table 5. Recommended Dose Reduction for Adverse Reactions for venetoclax in
CLL/SLL
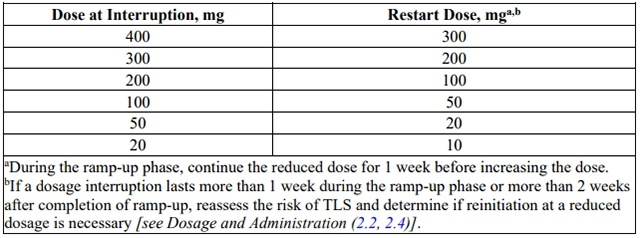
Table 6. Recommended venetoclax Dosage Modifications for Adverse Reactions in
AML
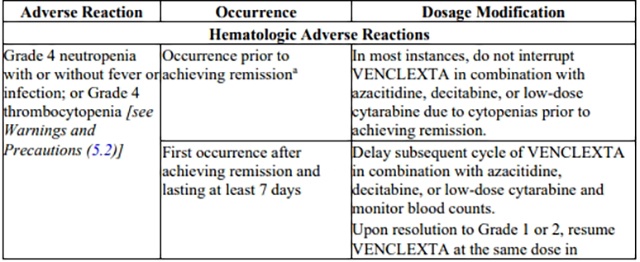
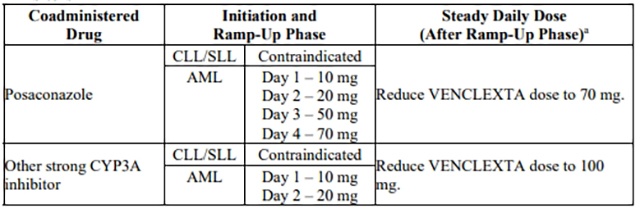
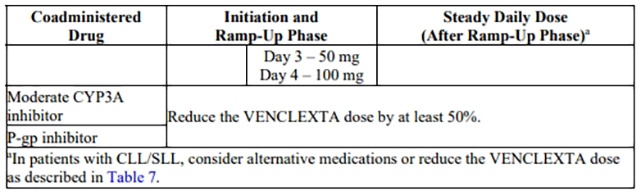
Table 7 describes venetoclax contraindication or dosage modification based on concomitant use with a strong or moderate CYP3A inhibitor or a P-gp inhibitor [see Drug Interactions (7.1)] at initiation, during, or after the ramp-up phase.
Reduce the venetoclax once daily dose by 50% for patients with severe hepatic impairment(Child-Pugh C); monitor these patients more closely for adverse reactions.
The recommended dosage of venetoclax may be delivered using any of the approved tablet strengths (e.g., patients can take 2 x 50 mg tablets or 10 x 10 mg tablets instead of 1 x 100 mg tablet as needed).
If the patient misses a dose of venetoclax within 8 hours of the time it is usually taken,instruct the patient to take the missed dose as soon as possible and resume the normal daily dosing schedule. If a patient misses a dose by more than 8 hours, instruct the patient not to take the missed dose and resume the usual dosing schedule the next day.If the patient vomits following dosing, instruct the patient to not take an additional dose that day and to take the next prescribed dose at the usual time.
from FDA,2022.06
Venetoclax is a small molecule drug approved in combination with azacitidine for···【more】
Release date:2024-08-13Recommended:236
Venetoclax is an oral targeted drug that can effectively inhibit the anti-apopto···【more】
Release date:2024-08-13Recommended:298
Venetoclax is a targeted drug used in the treatment of a variety of hematologic ···【more】
Release date:2024-08-13Recommended:252