The confirmed, investigator-assessed best overall response rate was 48.4% (95% CI: 41.6%, 55.2%) in the Vemurafenib arm compared to 5.5% (95% CI: 2.8%, 9.3%) in the dacarbazine arm. There were 2 complete responses (0.9%) and 104 partial responses (47.4%) in the Vemurafenib arm and all 12 responses were partial responses (5.5%) in the dacarbazine arm.
The confirmed best overall response rate as assessed by an independent review committee (IRC) was 52% (95% CI: 43%, 61%). There were 3 complete responses (2.3%) and 66 partial responses (50.0%). The median time to response was 1.4 months with 75% of responses occurring by month 1.6 of treatment. The median duration of response by IRC was 6.5 months (95% CI: 5.6, not reached).
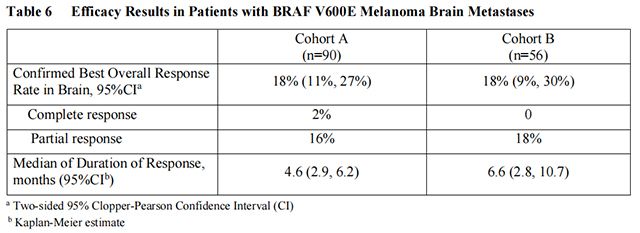
Vemurafenib has not been studied in patients with wild-type BRAF melanoma.
The efficacy of Vemurafenib in ECD was based on best overall response rate maintained on two occasions at least four weeks apart, as assessed by the investigator using RECIST v 1.1. The median duration of follow up was 26.6 months in ECD patients (range, 3.0 to 44.3 months). The median time to response was 11 months (95% CI: 3.7, 14.6). The median DOR was not estimable.
from FDA,2020.12