The efficacy and safety of Upadacitinib 15 mg once daily were assessed in five Phase 3 randomized, double-blind, multicenter trials in patients with moderately to severely active rheumatoid arthritis and fulfilling the ACR/EULAR 2010 classification criteria. Patients 18 years of age and older were eligible to participate. The presence of at least 6 tender and 6 swollen joints and evidence of systemic inflammation based on elevation of hsCRP was required at baseline. Although other doses have been studied, the recommended dosage of Upadacitinib is 15 mg once daily.
Clinical Response
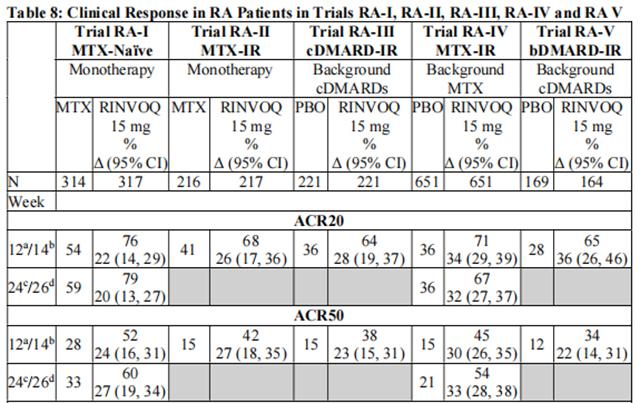
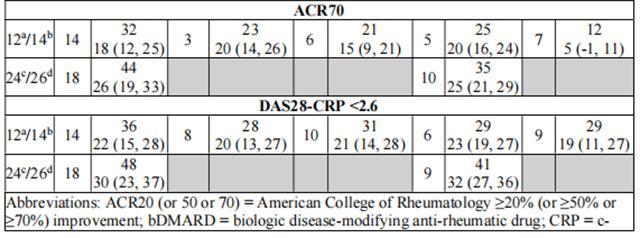
The percentages of Upadacitinib-treated patients achieving ACR20, ACR50, and ACR70 responses, and DAS28(CRP) < 2.6 in all trials are shown in Table 8. 
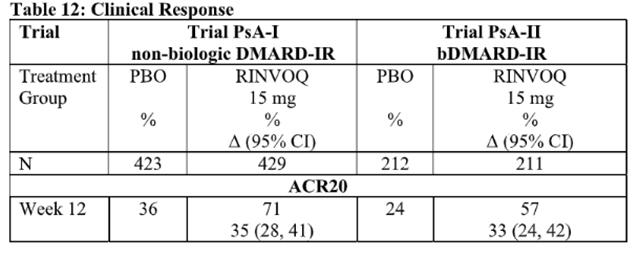
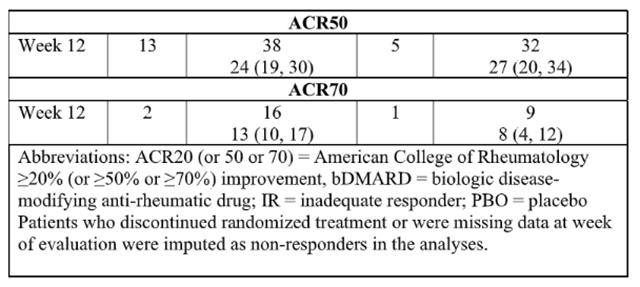
The efficacy and safety of Upadacitinib 15 mg once daily were assessed in two Phase 3 randomized, double-blind, multicenter, placebo-controlled trials in patients 18 years of age or older with moderately to severely active psoriatic arthritis. All patients had active psoriatic arthritis for at least 6 months based upon the Classification Criteria for Psoriatic Arthritis (CASPAR), at least 3 tender joints and at least 3 swollen joints, and active plaque psoriasis or history of plaque psoriasis. Although another dose has been studied, the recommended dose of Upadacitinib is 15 mg once daily for psoriatic arthritis.
Clinical Response
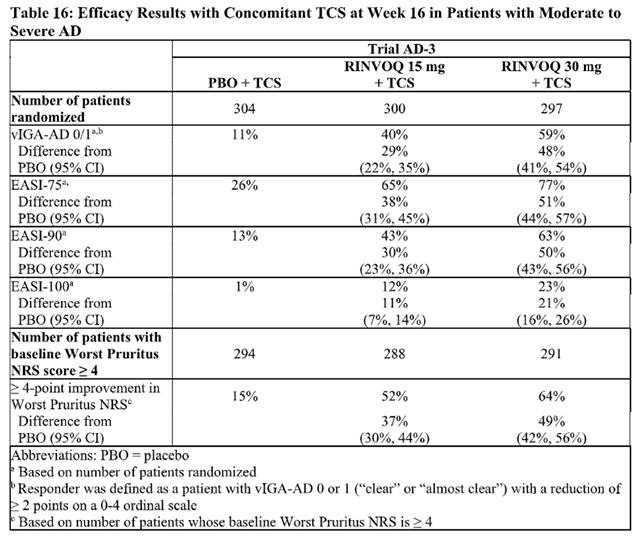
The efficacy of Upadacitinib 15 mg and 30 mg once daily, was assessed in three Phase 3 randomized, double-blind, multicenter trials (AD-1, AD-2, AD-3; NCT03569293, NCT03607422, and NCT03568318, respectively) in a total of 2584 patients (12 years of age and older). Upadacitinib was evaluated in 344 pediatric patients and 2240 adult patients with moderate to severe atopic dermatitis (AD) not adequately controlled by topical medication(s).
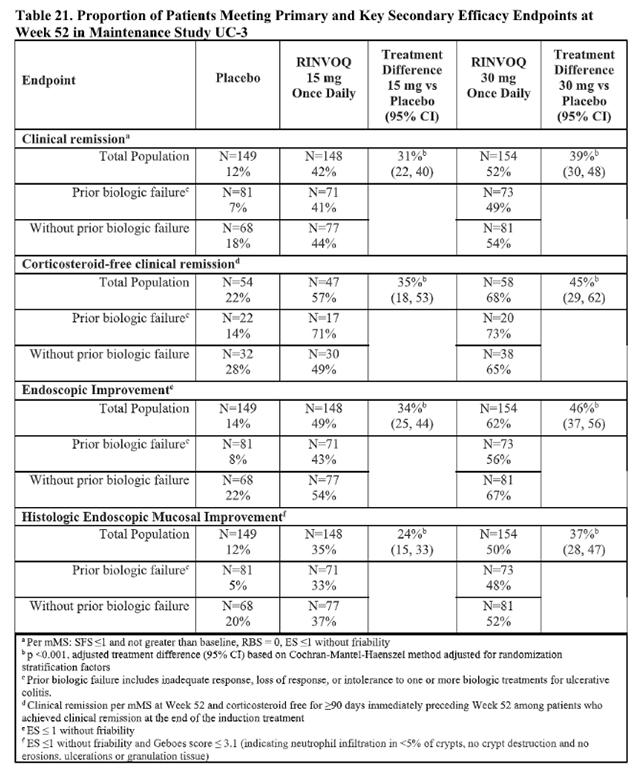
In two identical induction trials, patients were randomized 2:1 to receive either Upadacitinib 45 mg once daily or placebo for 8 weeks. A total of 988 patients were analyzed across the two trials. These trials included adult patients with moderately to severely active ulcerative colitis who had an inadequate response, loss of response, or intolerance to oral aminosalicylates, corticosteroids, immunosuppressants, and/or biologic therapy. Enrolled patients were permitted to use stable doses of oral aminosalicylates, methotrexate, ulcerative colitis-related antibiotics, and/or oral corticosteroids (up to 30 mg/day prednisone or equivalent). At baseline, 38% of patients were receiving corticosteroids, and 68% of patients were receiving aminosalicylates. Concomitant biologic therapies, azathioprine, 6-mercaptopurine, intravenous or rectal corticosteroids were prohibited. A total of 51% of patients had previously failed treatment with or were intolerant to at least one biologic therapy. Upadacitinib is indicated for patients who have an inadequate response or intolerance to one or more TNF blockers .
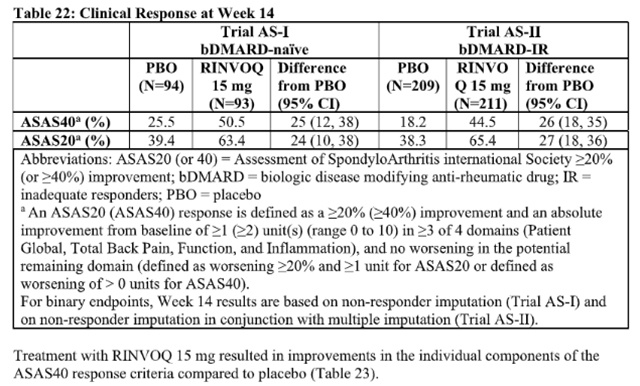
The efficacy and safety of Upadacitinib 15 mg once daily were assessed in two randomized, double-blind, multicenter, placebo-controlled trials in patients 18 years of age or older with active ankylosing spondylitis based upon the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ≥4 and Patient’s Assessment of Total Back Pain score ≥4.
Clinical Response
In both trials, a significantly greater proportion of patients treated with Upadacitinib 15 mg achieved an ASAS40 response compared to placebo at Week 14 (Table 22, Figure 5).
Examination of gender, baseline body mass index (BMI), and baseline hsCRP did not identify differences in response to Upadacitinib among these subgroups at Week 14.
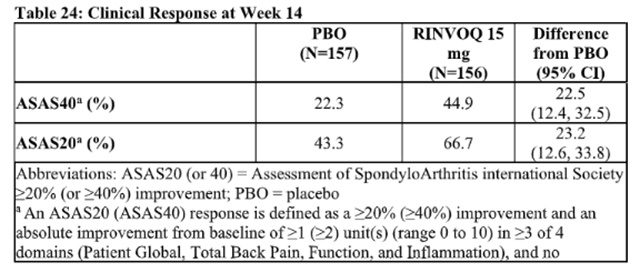
The efficacy and safety of Upadacitinib 15 mg once daily were assessed in a randomized, double-blind, multicenter, placebo-controlled trial in patients 18 years of age or older with active non-radiographic axial spondyloarthritis. Trial nr-axSpA (NCT04169373) was a 52-week placebo-controlled trial in 314 patients (of which 313 patients received study treatment) with active non-radiographic axial spondyloarthritis with an inadequate response to at least two nonsteroidal anti-inflammatory drugs (NSAIDs) or intolerance to or contraindication for NSAIDs. Patients must have had objective signs of inflammation indicated by elevated C-reactive protein (CRP) (defined as > upper limit of normal), and/or sacroiliitis on magnetic resonance imaging (MRI), and no definitive radiographic evidence of structural damage on sacroiliac joints. Patients had active disease as defined by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ≥4, and a Patient's Assessment of Total Back Pain score ≥ 4 based on a 0 – 10 numerical rating scale (NRS) at the Screening and Baseline Visits. At baseline, approximately 29.1% of the patients were on a concomitant cDMARD. 32.9% of the patients had an inadequate response or intolerance to bDMARD therapy. Patients received Upadacitinib 15 mg once daily or placebo. The primary endpoint was the proportion of patients achieving an Assessment of SpondyloArthritis international Society 40 (ASAS40) response at Week 14.
Clinical Response
In Trial nr-axSpA, a significantly greater proportion of patients treated with Upadacitinib 15 mg achieved an ASAS40 response compared to placebo at Week 14 (Table 24, Figure 6).
Examination of gender, baseline BMI, symptom duration of non-radiographic axial spondyloarthritis, baseline hsCRP, MRI sacroiliitis, and prior use of bDMARDs did not identify differences in response to Upadacitinib among these subgroups at Week 14.
from FDA,2023.04