Active and latent tuberculosis (TB) infection evaluation - If positive, treat for TB prior to Upadacitinib use.
Viral hepatitis screening in accordance with clinical guidelines - Upadacitinib initiation is not recommended in patients with active hepatitis B or hepatitis C.
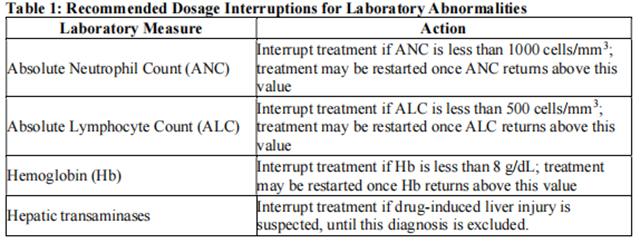
A complete blood count - Upadacitinib initiation is not recommended in patients with an absolute lymphocyte count less than 500 cells/mm3 , absolute neutrophil count less than 1000 cells/mm3 , or hemoglobin level less than 8 g/dL.
Upadacitinib initiation is not recommended for patients with severe hepatic impairment .
Verify the pregnancy status of females of reproductive potential prior to starting treatment.
Upadacitinib tablets should be taken orally with or without food.
Upadacitinib tablets should be swallowed whole. Upadacitinib should not be split, crushed, or chewed.
The recommended dosage of Upadacitinib is 15 mg once daily.
The recommended dosage of Upadacitinib is 15 mg once daily.
Initiate treatment with 15 mg once daily. If an adequate response is not achieved, consider increasing the dosage to 30 mg once daily. Discontinue Upadacitinib if an adequate response is not achieved with the 30 mg dose. Use the lowest effective dose needed to maintain response.
The recommended dosage is 15 mg once daily.
The recommended induction dose of Upadacitinib is 45 mg once daily for 8 weeks.
The recommended dose of Upadacitinib for maintenance treatment is 15 mg once daily. A dosage of 30 mg once daily may be considered for patients with refractory, severe or extensive disease.Discontinue Upadacitinib if an adequate therapeutic response is not achieved with the 30 mg dosage. Use the lowest effective dosage needed to maintain response.
The recommended dosage of Upadacitinib is 15 mg once daily.
The recommended dosage of Upadacitinib is 15 mg once daily.
No dosage adjustment is needed for patients with mild, moderate, or severe renal impairment.
For patients with severe renal impairment the recommended dosage is 15 mg once daily.
No dosage adjustment is needed for patients with mild or moderate renal impairment.
Upadacitinib is not recommended for use in patients with end stage renal disease.
For patients with severe renal impairment (eGFR 15 to < 30 mL/min/1.73m2 ), the recommended dosage is: Induction: 30 mg once daily for 8 weeks,15 mg once daily.
No dosage adjustment is needed for patients with mild or moderate renal impairment.
Upadacitinib is not recommended for use in patients with end stage renal disease
Upadacitinib is not recommended for use in patients with severe hepatic impairment.
No dosage adjustment is needed for patients with mild or moderate hepatic impairment.
For patients with mild to moderate hepatic impairment the recommended dosage is 30 mg once daily for 8 weeks,15 mg once daily.
The recommended dosage in patients receiving strong CYP3A4 inhibitors is 15 mg once daily.
The recommended dosage in patients with ulcerative colitis receiving strong CYP3A4 inhibitors 30 mg once daily for 8 weeks,15 mg once daily.
If a patient develops a serious infection, including serious opportunistic infection, interrupt Upadacitinib treatment until the infection is controlled.
Interruption of dosing may be needed for management of laboratory abnormalities as described in Table 1
from FDA,2023.04