Select patients for treatment of unresectable or metastatic colorectal cancer with Tucatinib based on the presence of:
HER2 overexpression or gene amplification. FDA-approved tests for the detection of HER2 overexpression and gene amplification in patients with unresectable or metastatic colorectal cancer are not currently available, and RAS wild-type.
The recommended dosage of Tucatinib is 300 mg taken orally twice daily in combination with trastuzumab and capecitabine until disease progression or unacceptable toxicity.
The recommended dosage of Tucatinib is 300 mg taken orally twice daily in combination with trastuzumab until disease progression or unacceptable toxicity.
Advise patients to swallow Tucatinib tablets whole and not to chew, crush, or split prior to swallowing. Advise patients not to ingest tablet if it is broken, cracked, or not otherwise intact.
Advise patients to take Tucatinib approximately 12 hours apart and at the same time each day with or without a meal.
If the patient vomits or misses a dose of Tucatinib, instruct the patient to take the next dose at its usual scheduled time.
When given in combination with Tucatinib, the recommended dosage of capecitabine is 1000 mg/m2 orally twice daily taken within 30 minutes after a meal. Tucatinib and capecitabine can be taken at the same time. Refer to the Full Prescribing Information for trastuzumab and capecitabine for additional information.
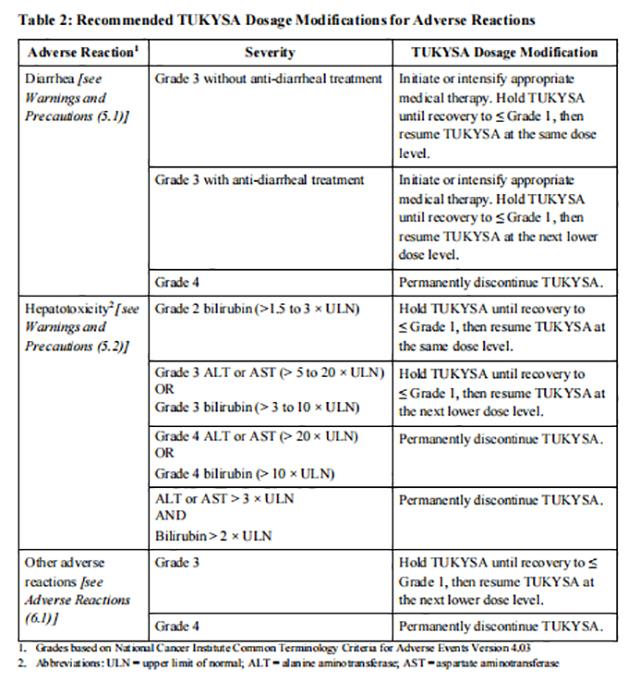
The recommended Tucatinib dose reductions and dosage modifications for adverse reactions are provided in Tables 1 and 2. Refer to the Full Prescribing Information for trastuzumab and capecitabine for information about dosage modifications for these drugs.
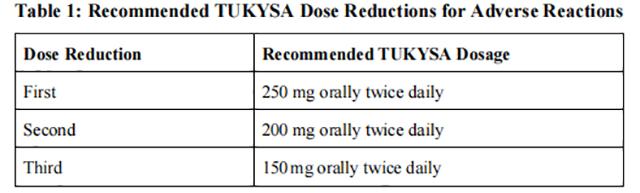
Permanently discontinue Tucatinib in patients unable to tolerate 150 mg orally twice daily.
For patients with severe hepatic impairment (Child-Pugh C), reduce the recommended dosage to 200 mg orally twice daily.
Avoid concomitant use of strong CYP2C8 inhibitors with Tucatinib. If concomitant use with a strong CYP2C8 inhibitor cannot be avoided, reduce the recommended dosage to 100 mg orally twice daily. After discontinuation of the strong CYP2C8 inhibitor for 3 elimination half-lives, resume the Tucatinib dose that was taken prior to initiating the inhibitor.
from FDA,2023.01