Confirm the presence of BRAF V600E or V600K mutation in tumor specimens prior to initiation of treatment with trametinib as a single agent or in combination with dabrafenib.
NSCLC
Confirm the presence of BRAF V600E mutation in tumor specimens prior to initiation of treatment with trametinib and dabrafenib.
ATC
Confirm the presence of BRAF V600E mutation in tumor specimens prior to initiation of treatment with trametinib and dabrafenib. An FDA-approved test for the detection of BRAF V600E mutation in ATC is not currently available.
Solid Tumors
Confirm the presence of BRAF V600E mutation in tumor specimens prior to initiation of treatment with trametinib and dabrafenib. An FDA-approved test for the detection of BRAF V600E mutation in solid tumors other than melanoma and NSCLC is not currently available.
Low-Grade Glioma
Confirm the presence of BRAF V600E mutation in tumor specimens prior to initiation of treatment with trametinib and dabrafenib. An FDA-approved test for the detection of BRAF V600E mutation in LGG is not currently available.
Adult Patients
The recommended dosage for trametinib tablets in adult patients is 2 mg orally taken once daily.
Pediatric Patients
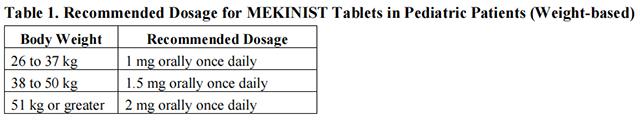
The recommended dosage for trametinib tablets in pediatric patients who weigh at least 26 kg is based on body weight (Table 1). A recommended dosage of trametinib tablets has not been established in patients who weigh less than 26 kg.
Trametinib for Oral Solution
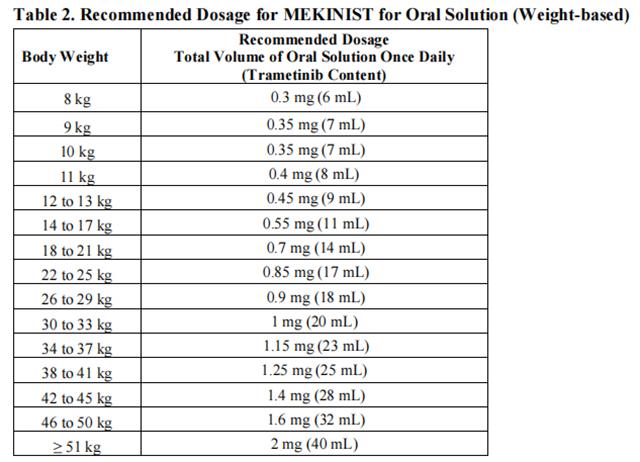
The recommended dosage for trametinib for oral solution is based on body weight (Table 2).
The recommended duration of treatment for patients with unresectable or metastatic melanoma or solid tumors, metastatic NSCLC, or locally advanced or metastatic anaplastic thyroid cancer is until disease progression or unacceptable toxicity.
The recommended duration of treatment in the adjuvant melanoma setting is until disease recurrence or unacceptable toxicity for up to 1 year.
The recommended duration of treatment for pediatric patients with LGG is until disease progression or until unacceptable toxicity.
Take trametinib at the same time each day, approximately 24 hours apart.
Take trametinib at least 1 hour before or 2 hours after a meal.
Do not take a missed dose of trametinib within 12 hours of the next dose of trametinib.
If vomiting occurs after trametinib administration, do not take an additional dose. Take the next dose at its scheduled time.
Trametinib Tablets
Do not crush or break trametinib tablets.
Trametinib for Oral Solution
trametinib for oral solution is intended for administration by a caregiver. Prior to use of the oral solution, ensure caregivers receive training on proper dosing and administration of trametinib for oral solution.
To prepare trametinib for oral solution, tap the bottle until powder flows freely. Add 90 mL distilled or purified water to the powder in the bottle and invert or gently shake the bottle with re-attached cap for up to 5 minutes until powder is fully dissolved yielding a clear solution. Separate the bottle adapter from the oral syringe. Insert bottle adapter into bottle neck after reconstitution of the solution. Write the discard after date.
Once reconstituted, trametinib for oral solution can be used for 35 days.
The final concentration of the solution is 0.05 mg/mL.
Administer trametinib for oral solution from an oral syringe or feeding tube (4 French gauge or larger).
After reconstitution, store in original bottle below 25°C (77°F) and do not freeze.
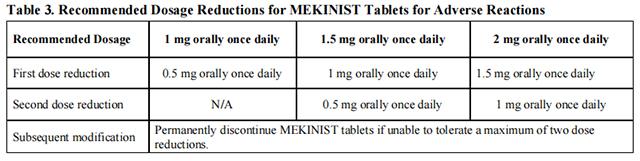
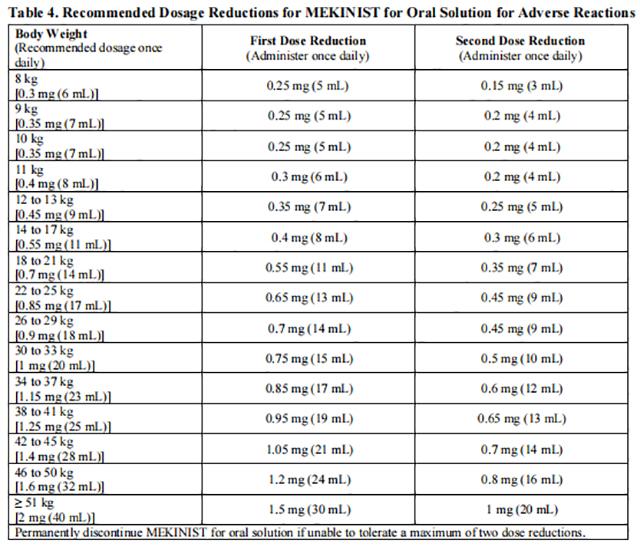
Dose reductions for adverse reactions associated with trametinib are presented in Tables 3 and 4.
Dosage modifications for adverse reactions associated with trametinib are presented in Table 5.
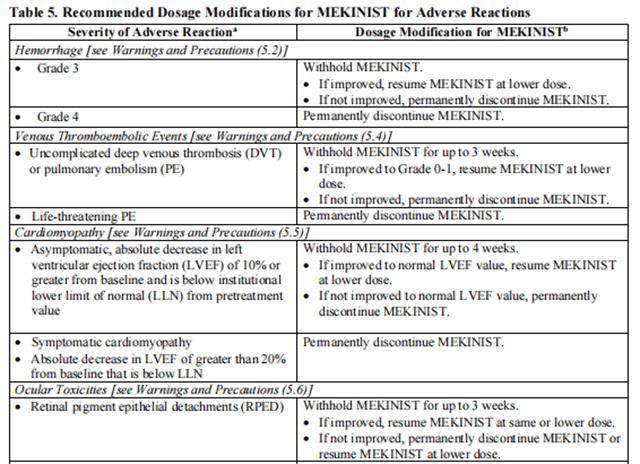
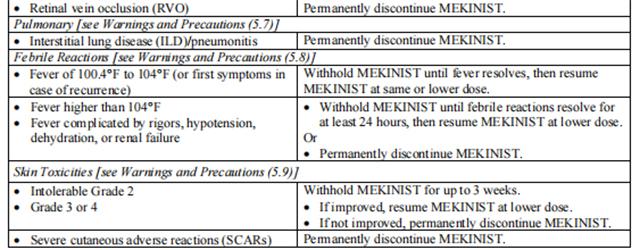
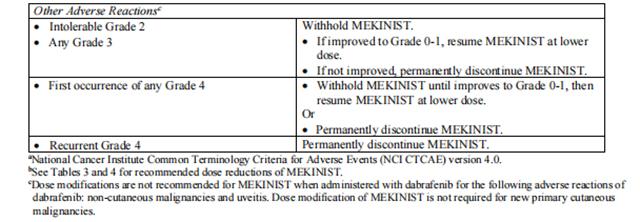
Refer to the dabrafenib prescribing information for dose modifications for adverse reactions associated with dabrafenib.
from FDA,2024.03
Trametinib is a targeted drug used to treat specific types of cancer, and its do···【more】
Release date:2025-01-08Recommended:241
Trametinib is a targeted therapy drug that has attracted much attention in clini···【more】
Release date:2025-01-08Recommended:226