The efficacy of Tivozanib was evaluated in TIVO-3 (NCT02627963), a randomized (1:1), open-label, multicenter trial of Tivozanib versus sorafenib in patients with relapsed or refractory advanced RCC who received 2 or 3 prior systemic treatments including at least one VEGFR kinase inhibitor other than sorafenib or tivozanib. Patients were randomized to receive Tivozanib 1.34 mg orally once daily for 21 days on treatment followed by 7 days off treatment for a 28-day cycle, or to receive sorafenib 400 mg orally twice a day continuously, until disease progression or unacceptable toxicity. Randomization was stratified by prior therapy [two kinase inhibitors (KIs), a KI plus an immune checkpoint inhibitor, or a KI plus other systemic agents] and by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) prognostic score. Patients were excluded if they had more than 3 prior treatments or Central Nervous System metastases.
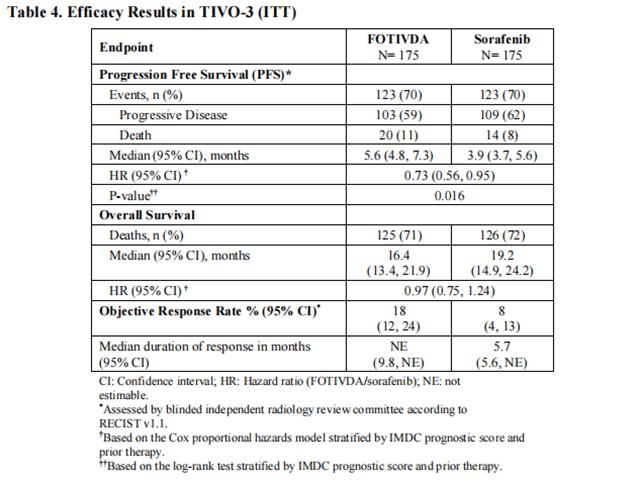
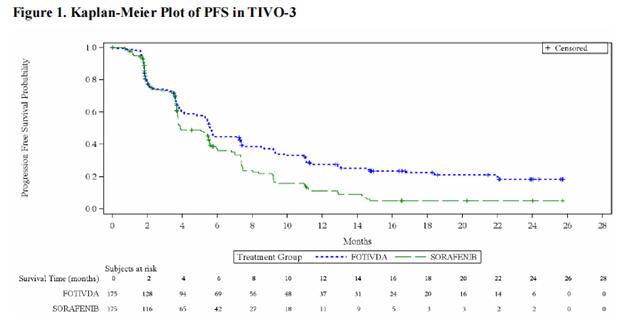
The main efficacy outcome measure was progression-free survival (PFS) assessed by a blinded independent radiology review committee. Other efficacy endpoints were objective response rate (ORR) and overall survival (OS). The median age was 63 years (range: 30 to 90 years), 73% were male, 95% were Caucasian, ECOG performance status was 0 in 48% and 1 in 49% of patients (respectively), and 98% of patients had clear cell or clear cell component histology. Prior therapy included two KIs (45%), a KI plus an immune checkpoint inhibitor (26%), and a KI plus another systemic agent (29%). At the time of study entry, 20% of patients had favorable, 61% intermediate, and 19% poor IMDC prognoses.
Efficacy results are summarized in Table 4 and Figure 1.
from FDA,2021.03