Select patients for treatment with Tepotinib based on the presence of MET exon 14 skipping alterations in plasma or tumor specimens. Testing for the presence of MET exon 14 skipping alterations in plasma specimens is recommended only in patients for whom a tumor biopsy cannot be obtained. If an alteration is not detected in a plasma specimen, re-evaluate the feasibility of biopsy for tumor tissue testing. An FDA-approved test for detection of MET exon 14 skipping alterations in NSCLC for selecting patients for treatment with Tepotinib is not available.
The recommended dosage of Tepotinib is 450 mg orally once daily with food until disease progression or unacceptable toxicity.
Instruct patients to take their dose of Tepotinib at approximately the same time every day and to swallow tablets whole.
Do not chew, crush or split tablets. Advise patients not to make up a missed dose within 8 hours of the next scheduled dose.
If vomiting occurs after taking a dose of Tepotinib, advise patients to take the next dose at the scheduled time.
Place Tepotinib tablet(s) in a glass containing 30 mL (1 ounce) of non-carbonated water. No other liquids should be used or added. Stir, without crushing, until the tablet(s) is dispersed into small pieces (tablets will not completely dissolve) and drink immediately or within 1 hour. Swallow the tablet dispersion. Do not chew pieces of the tablet. Rinse the glass with an additional 30 mL and drink immediately ensuring no residue remains in the glass and the full dose is administered.
If an administration via a naso-gastric tube (with at least 8 French gauge) is required, disperse the tablet(s) in 30 mL of non-carbonated water as described above. Administer the 30 mL of liquid immediately or within 1 hour as per naso-gastric tube manufacturer’s instructions. Immediately rinse twice with 30 mL each time to ensure that no residue remains in the glass or syringe and the full dose is administered.
The recommended dose reduction of Tepotinib for the management of adverse reactions is 225 mg orally once daily. Permanently discontinue Tepotinib in patients who are unable to tolerate 225 mg orally once daily.
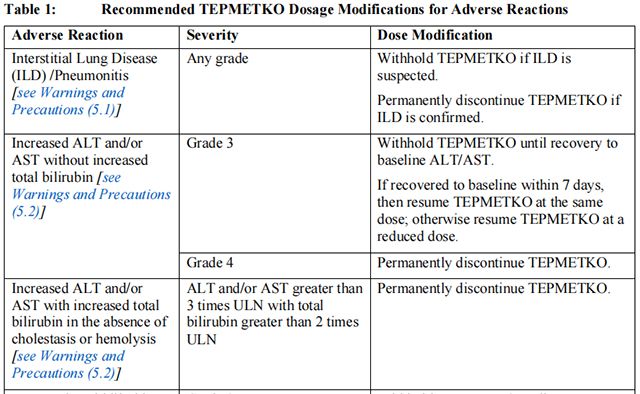
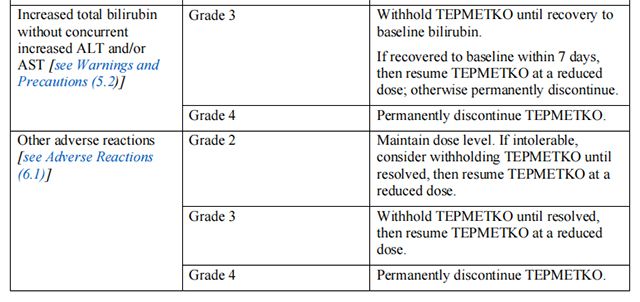
The recommended dosage modifications of Tepotinib for adverse reactions are provided in Table 1.
from FDA,2023.03