The efficacy of LUMAKRAS was demonstrated in a subset of patients enrolled in a single-arm, open-label, multicenter trial (CodeBreaK 100 [NCT03600883]). Eligible patients were required to have locally advanced or metastatic KRAS G12C-mutated NSCLC with disease progression after receiving an immune checkpoint inhibitor and/or platinum-based chemotherapy, an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1, and at least one measurable lesion as defined by Response Evaluation Criteria in Solid Tumors (RECIST v1.1).
All patients were required to have prospectively identified KRAS G12C-mutated NSCLC in tumor tissue samples by using the QIAGEN therascreen® KRAS RGQ PCR Kit performed in a central laboratory. Of 126 total enrolled subjects, 2 (2%) were unevaluable for efficacy analysis due to the absence of radiographically measurable lesions at baseline. Of the 124 patients with KRAS G12C mutations confirmed in tumor tissue, plasma samples from 112 patients were tested retrospectively using the Guardant360® CDx. 78/112 patients (70%) had KRAS G12C mutation identified in plasma specimen, 31/112 patients (28%) did not have KRAS G12C mutation identified in plasma specimen and 3/112 (2%) were unevaluable due to Guardant360® CDx test failure.
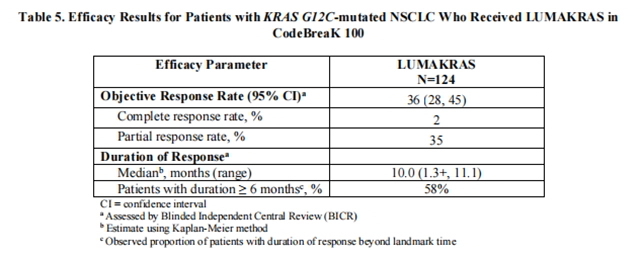
A total of 124 patients had at least one measurable lesion at baseline assessed by Blinded Independent Central Review (BICR) according to RECIST v1.1 and were treated with LUMAKRAS 960 mg once daily until disease progression or unacceptable toxicity. The major efficacy outcome measures were objective response rate (ORR) and duration of response (DOR) as evaluated by BICR according to RECIST v1.1.
The baseline demographic and disease characteristics of the study population were: median age 64 years (range: 37 to 80) with 48% ≥ 65 years and 8% ≥ 75 years; 50% Female; 82% White, 15% Asian, 2% Black; 70% ECOG PS 1; 96% had stage IV disease; 99% with non-squamous histology; 81% former smokers, 12% current smokers, 5% never smokers. All patients received at least 1 prior line of systemic therapy for metastatic NSCLC; 43% received only 1 prior line of therapy, 35% received 2 prior lines of therapy, 23% received 3 prior lines of therapy; 91% received prior anti-PD-1/PD-L1 immunotherapy, 90% received prior platinum-based chemotherapy, 81% received both platinum-based chemotherapy and anti-PD-1/PD-L1. The sites of known extra-thoracic metastasis included 48% bone, 21% brain, and 21% liver.
Efficacy results are summarized in Table 5.
from FDA,2023.04
In a phase 1 study, sotorasib showed anticancer activity in patients with advanc···【more】
Release date:2024-11-15Recommended:182
Sotorasib, a novel targeted medication designed specifically for patients with N···【more】
Release date:2024-08-05Recommended:227
Sotorasib is a targeted therapy for patients with advanced NSCLC with KRAS p.G12···【more】
Release date:2024-07-09Recommended:347