FIGHT-202 (NCT02924376), a multicenter open-label single-arm trial, evaluated the efficacy of selpercatinib in 107 patients with locally advanced unresectable or metastatic cholangiocarcinoma whose disease had progressed on or after at least 1 prior therapy and who had an FGFR2 gene fusion or non-fusion rearrangement, as determined by a clinical trial assay performed at a central laboratory. Qualifying in-frame fusions and other rearrangements were predicted to have a breakpoint within intron 17/exon 18 of the FGFR2 gene leaving the FGFR2 kinase domain intact.
Patients received selpercatinib in 21-day cycles at a dosage of 13.5 mg orally once daily for 14 consecutive days, followed by 7 days off therapy. selpercatinib was administered until disease progression or unacceptable toxicity. The major efficacy outcome measures were overall response rate (ORR) and duration of response (DoR) as determined by an independent review committee (IRC) according to RECIST v1.1.
The median age was 56 years (range: 26 to 77 years), 61% were female, 74% were White, and 95% had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of (42%) or 1 (53%). Ninety-eight percent of patients had intrahepatic cholangiocarcinoma.Eighty-six percent of patients had in-frame FGFR2 gene fusions and the most commonly identified FGFR2 fusion was FGFR2-BICC1 (34%). Fourteen percent of patients had other FGFR2 rearrangements that could not be confidently predicted to be in-frame fusions, including rearrangements without an identifiable partner gene. All patients had received at least 1 prior line of systemic therapy, 27% had 2 prior lines of therapy, and 12% had 3 or more prior lines of therapy. Ninety-six percent of patients had received prior platinum-based therapy including 76% with prior gemcitabine/cisplatin.
Efficacy results are summarized in Table 7.
The median time to response was 2.7 months (range 0.7 – 6.9 months).
Table 7: Efficacy Results in FIGHT-202
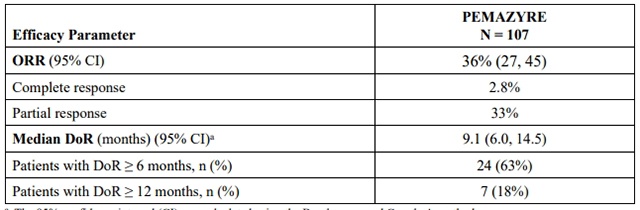
a The 95% confidence interval (CI) was calculated using the Brookmeyer and Crowley's method.
Note: Data are from IRC per RECIST v1.1, and complete and partial responses are confirmed.
from FDA,2024.06
In recent years, cerpatinib has gained a lot of attention as a drug to treat spe···【more】
Release date:2024-12-25Recommended:261
In recent years, cerpatinib is a new type of drug that has attracted much attent···【more】
Release date:2024-12-25Recommended:199
As a new type of therapeutic drug, cerpatinib has attracted much attention in re···【more】
Release date:2024-12-25Recommended:257
Selpercatinib is the first targeted drug specifically targeting RET gene mutatio···【more】
Release date:2024-08-16Recommended:183
In May 2020, Selpercatinib was approved by the FDA for the treatment of various ···【more】
Release date:2024-08-16Recommended:252
Selpercatinib treats cancer patients by specifically inhibiting RET kinase activ···【more】
Release date:2024-08-16Recommended:207