The efficacy of Repotrectinib was evaluated in TRIDENT-1, a multicenter, single-arm, open-label, multi-cohort clinical trial (NCT03093116).
Efficacy results are summarized in Table 5.
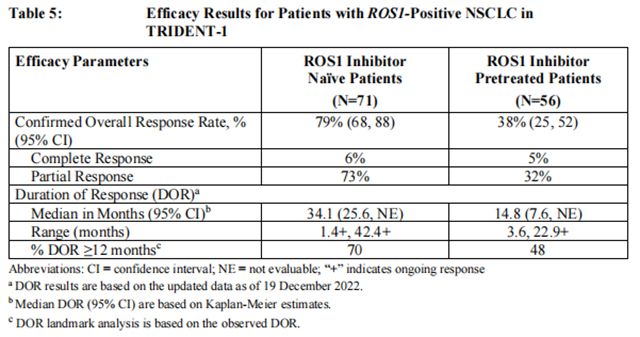
Among TKI-naïve patients, 8 had measurable CNS metastases at baseline as assessed by BICR; responses in intracranial lesions were observed in 7 of these 8 patients. Among the TKI pretreated patients with no prior platinum-based chemotherapy, 12 had measurable CNS metastases at baseline as assessed by BICR; responses in intracranial lesions were observed in 5 of these 12 patients.
Among the 56 ROS1 inhibitor-pretreated patients, 8 had resistance mutations following TKI therapy. Responses were observed in 6 of these 8 patients; responders included patients with solvent front (G2032R), gatekeeper (L2026M), and other mutations (S1986F/Y).
from FDA,2023.11