Select patients for the treatment of locally advanced or metastatic NSCLC with Repotrectinib based on the presence of ROS1 rearrangement(s) in tumor specimens. An FDA-approved test to detect ROS1 rearrangements for selecting patients for treatment with Repotrectinib is not currently available.
Prior to initiating Repotrectinib, discontinue strong and moderate CYP3A inhibitors for 3 to 5 elimination half-lives of the CYP3A inhibitor.
Prior to initiation of Repotrectinib, evaluate:
liver function tests including bilirubin.
uric acid level.
The recommended dosage of Repotrectinib is 160 mg taken orally once daily with or without food for 14 days, then increase to 160 mg twice daily and continue until disease progression or unacceptable toxicity.
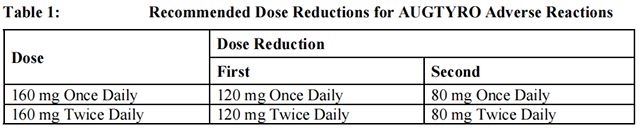
The recommended dosage reductions of Repotrectinib for the management of adverse reactions are provided in Table 1.
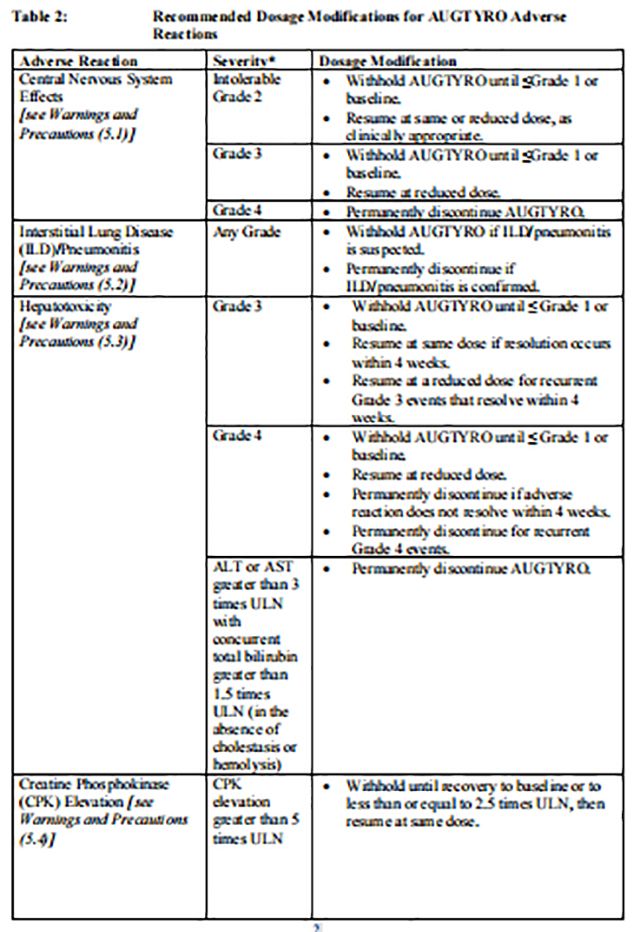
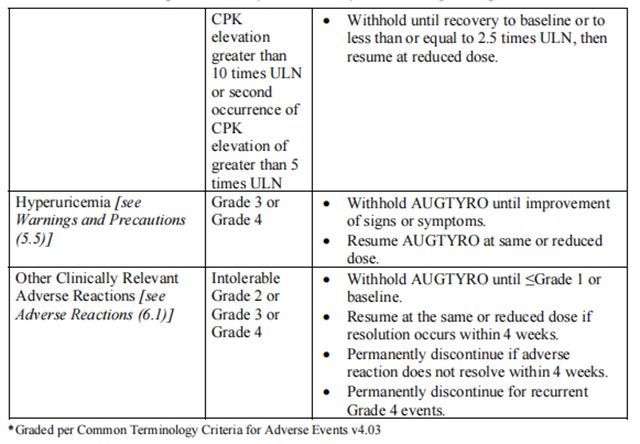
Recommended dosage modifications of Repotrectinib for the management of adverse reactions are provided in Table 2.
Take Repotrectinib at approximately the same time each day with or without food.
Swallow Repotrectinib capsules whole. Do not open, chew, crush, or dissolve the capsule prior to swallowing. Do not take any Repotrectinib capsules that are broken, cracked, or damaged.
If a dose of Repotrectinib is missed or if vomiting occurs at any time after taking a dose, skip the dose and resume Repotrectinib at its regularly scheduled time.
from FDA,2023.11