The efficacy of Larotrectinib was evaluated in pediatric and adult patients with unresectable or metastatic solid tumors with a NTRK gene fusion enrolled in one of three multicenter, open-label, single-arm clinical trials: Study LOXO-TRK-14001 (NCT02122913), SCOUT (NCT02637687), and NAVIGATE (NCT02576431). All patients were required to have progressed following systemic therapy for their disease, if available, or would have required surgery with significant morbidity for locally advanced disease.
Adult patients received Larotrectinib 100 mg orally twice daily and pediatric patients (18 years or younger) received Larotrectinib 100 mg/m2 up to a maximum dose of 100 mg orally twice daily until unacceptable toxicity or disease progression. Identification of positive NTRK gene fusion status was prospectively determined in local laboratories using next generation sequencing (NGS) or fluorescence in situ hybridization (FISH). NTRK gene fusions were inferred in three patients with infantile fibrosarcoma who had a documented ETV6 translocation identified by FISH. The major efficacy outcome measures were overall response rate (ORR) and duration of response (DOR), as determined by a blinded independent review committee (BIRC) according to RECIST v1.1.
The assessment of efficacy was based on the first 55 patients with solid tumors with an NTRK gene fusion enrolled across the three clinical trials. Baseline characteristics were: median age 45 years (range 4 months to 76 years); 22% <18 years of age, and 78% ≥18 years of age; 53% male; 67% White; 7% Hispanic/Latino, 4% Asian, 4% Black; and ECOG performance status (PS) 0-1 (93%) or 2 (7%). Eighty-two percent of patients had metastatic disease, including patients with brain metastases, and 18% had locally advanced, unresectable disease. Ninety-eight percent of patients had received prior treatment for their cancer, including surgery, radiotherapy, or systemic therapy. Of these, 82% (n = 45) received prior systemic therapy with a median of two prior systemic regimens and 35% (n = 19) received three or more prior systemic regimens. The most common cancers were salivary gland tumors (22%), soft tissue sarcoma (20%), infantile fibrosarcoma (13%), and thyroid cancer (9%). A total of 50 patients had NTRK gene fusions detected by NGS and 5 patients had NTRK gene fusions detected by FISH.
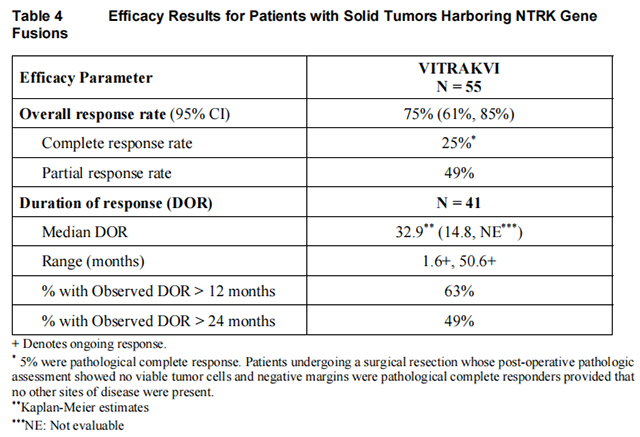
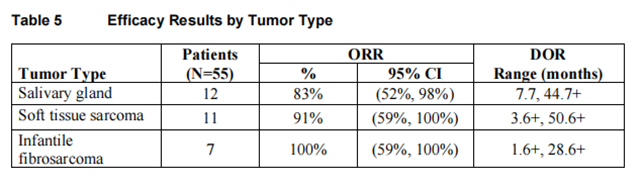
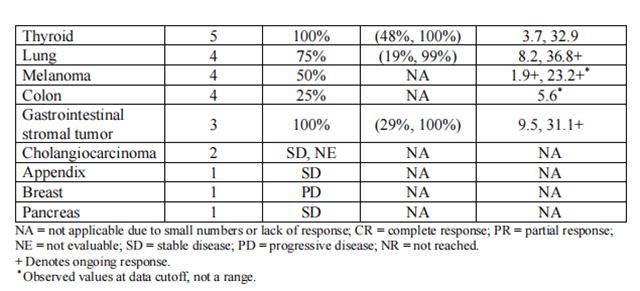
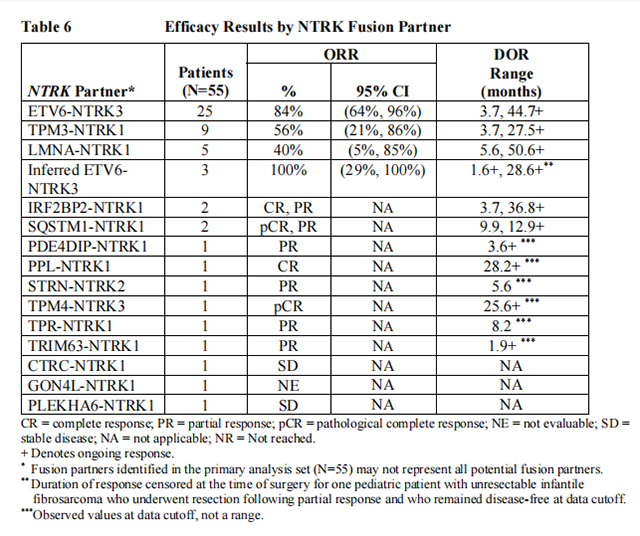
Efficacy results are summarized in Tables 4, 5, and 6.
from FDA,2022.11