The efficacy of Exkivity was evaluated in a pooled subset of patients with EGFR exon 20 insertion mutation-positive metastatic or locally advanced NSCLC whose disease had progressed on or after platinum-based chemotherapy enrolled in an international, open-label, multicohort clinical trial (AP32788-15-101, NCT02716116). Patients had histologically or cytologically confirmed locally advanced or metastatic disease (Stage IIIB or IV) and a documented EGFR exon 20 insertion mutation based on local testing. Patients received Exkivity at a dose of 160 mg once daily until disease progression or intolerable toxicity.
In the efficacy population, EGFR exon 20 insertion mutation status was determined by prospective local testing using samples from tumor tissue (87%), plasma (5%), or other specimens such as pleural fluid (8%). Of the 114 patients with EGFR exon 20 insertion mutations, 70% of patient tissue samples were tested retrospectively using Life Technologies Corporation Oncomine Dx™ Target Test. While 75% of patients were positive for EGFR exon 20 insertion mutation, 14% did not have an EGFR exon 20 insertion mutation identified, and 11% did not generate reportable results.
The efficacy population consisted of 114 patients and had the following demographic characteristics: the median age was 60 years (range: 27 to 84 years); 66% were female; 60% were Asian, 37% were White, and 3% were Black; 71% had never smoked; at baseline, 75% had Eastern Cooperative Oncology Group (ECOG) performance status 1. At baseline, 99% of patients had metastatic disease, 98% of patients had adenocarcinoma histology and 35% of patients had brain metastases. The median number of prior therapies was 2 (range: 1 to 7) and 43% percent had received prior immunotherapy.
The major efficacy outcome measure was overall response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1) as evaluated by blinded independent central review (BICR). Additional efficacy outcome measures included duration of response (DOR) by BICR.
Efficacy results are summarized in Table 5.
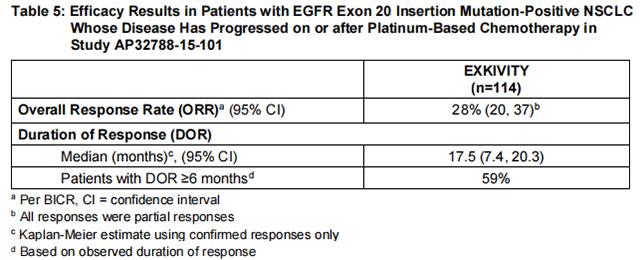
Investigator-assessed ORR was 35% (95% CI: 26, 45) with a median DOR of 11.2 months (63% of these patients had observed responses lasting longer than 6 months).
from FDA,2023.09
Is the Lao Lucius version of Exkivity effective?This product is indicated for th···【more】
Release date:2024-11-29Recommended:333
Exkivity is a broad-spectrum inhibitor that broadly inhibits multiple mutant kin···【more】
Release date:2024-08-08Recommended:244
Exkivity is a new type of targeted therapy for lung cancer, which is a third rep···【more】
Release date:2024-08-08Recommended:246
Exkivity is a novel oral anti-cancer drug that acts as a small molecule tyrosine···【more】
Release date:2024-08-08Recommended:266