The efficacy of Entrectinib was evaluated in a pooled subgroup of patients with ROS1-positive metastatic NSCLC who received Entrectinib at various doses and schedules (90% received Entrectinib 600 mg orally once daily) and were enrolled in one of three multicenter, single-arm, open-label clinical trials: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267).
Among the 92 patients, 10 had measurable CNS metastases at baseline as assessed by BICR and had not received radiation therapy to the brain within 2 months prior to study entry. Responses in intracranial lesions were observed in 7 of these 10 patients.
The efficacy of Entrectinib was evaluated in a pooled subgroup of adult patients with unresectable or metastatic solid tumors with a NTRK gene fusion enrolled in one of three multicenter, single-arm, open-label clinical trials: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267).
Among the subset of patients who received prior systemic therapy for metastatic disease, the ORR was 53%, similar to that seen in the overall population.
Among the 54 adult patients, 4 had measurable CNS metastases at baseline as assessed by BICR and had not received radiation therapy to the brain within 2 months of study entry. Responses in intracranial lesions were observed in 3 of these 4 patients.
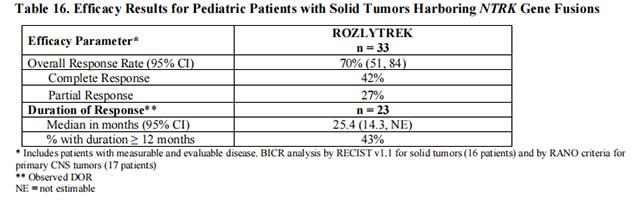
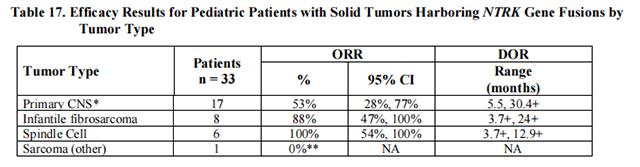
The efficacy of Entrectinib was evaluated in pediatric patients with unresectable or metastatic solid tumors with a NTRK gene fusion enrolled in one of two multicenter, open-label clinical trials: STARTRK-NG (NCT02650401) and TAPISTRY (NCT04589845).
Efficacy results are summarized in Tables 16 and 17.

from FDA,2024.01