Select patients for the treatment of metastatic NSCLC with Entrectinib based on the presence of ROS1 rearrangement(s) in tumor or plasma specimens. Testing using plasma specimens is only appropriate for patients for whom tumor tissue is not available for testing.
Select patients for treatment of locally advanced or metastatic solid tumors with Entrectinib based on the presence of a NTRK gene fusion in tumor or plasma specimens. Testing using plasma specimens is only appropriate for patients for whom tumor tissue is not available for testing.
Before initiating Entrectinib, evaluate:
left ventricular ejection fraction (LVEF)
serum uric acid levels
QT interval and electrolytes
The physician should prescribe the most appropriate dosage form of Entrectinib according to the dose required and patient needs.
Whole capsules: For patients who can swallow whole capsules and whose doses are multiples of 100 mg.
Capsules prepared as an oral suspension:
For patients who have difficulty or are unable to swallow capsules or who require enteral administration (e.g., gastric or nasogastric tube).
For dose increments of 10 mg, only use capsules prepared as a suspension.
Administer Entrectinib capsules, capsules prepared as a suspension, or pellets once daily, with or without food.
If a dose of ROZYTREK is missed, make up that dose unless the next dose is due within 12 hours.
If vomiting occurs immediately after taking a dose of Entrectinib, repeat that dose.
The recommended dosage of Entrectinib is 600 mg orally once daily with or without food until disease progression or unacceptable toxicity.
The recommended dosages of Entrectinib for the treatment of adult and pediatric patients with NTRK Gene Fusion-Positive Solid Tumors are provided in Table 1.
Administer the recommended dosage of Entrectinib capsules and oral pellets with or without food until disease progression or unacceptable toxicity.
Sprinkle Entrectinib oral pellets on one or more spoonfuls of soft food as a vehicle.
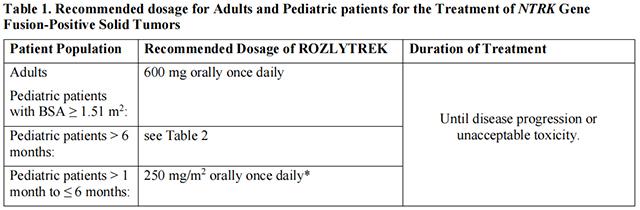
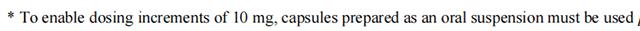
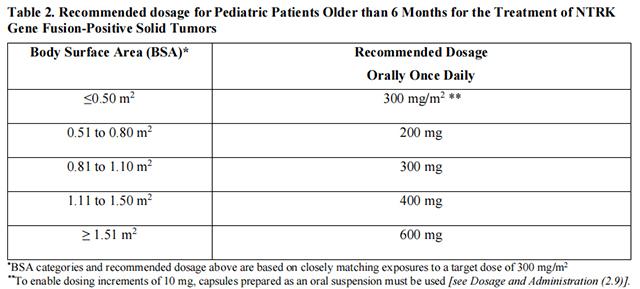
The recommended dosages of Entrectinib for the treatment of pediatric patients older than 6 months with NTRK Gene Fusion-Positive Solid Tumors is provided in Table 2.
The recommended dosage reductions of Entrectinib for the management of adverse reactions for adults and pediatric patients are provided in Table
The recommended dosage reductions of Entrectinib for the management of adverse reactions for adults and pediatric patients are provided in Table 3.
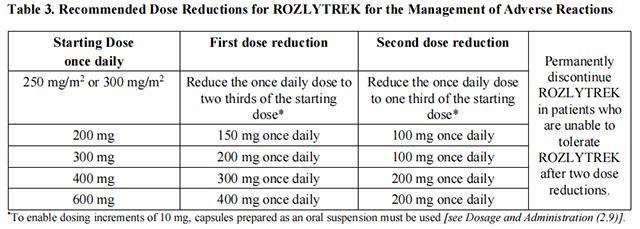
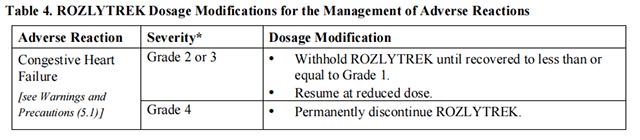
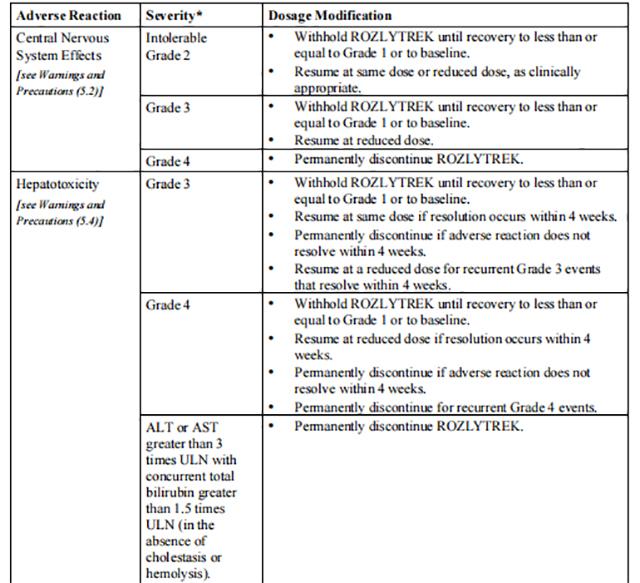
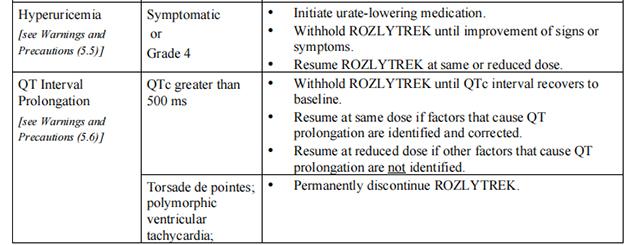
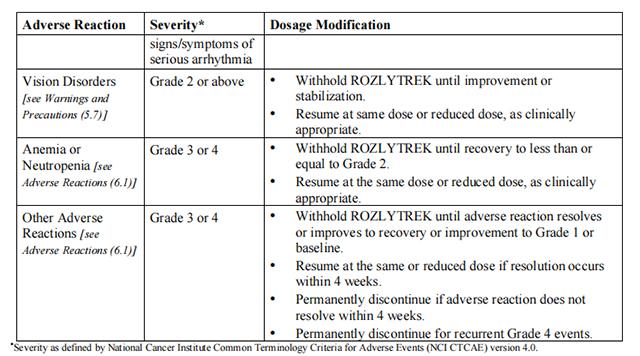
Table 4 provides the Entrectinib recommended dosage modifications for the management of adverse reactions.
Adults and Pediatric Patients 2 Years and Older
Avoid coadministration of Entrectinib with moderate or strong CYP3A inhibitors. If coadministration cannot be avoided, reduce the Entrectinib dose as shown in Table 5 and limit coadministration to 14 days or less.
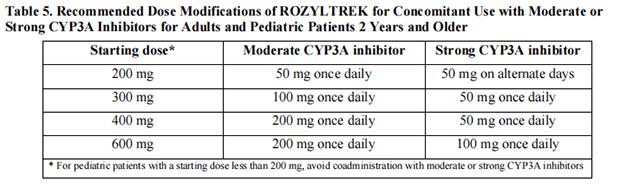
After discontinuation of a strong or moderate CYP3A inhibitor for 3 to 5 elimination half-lives, resume the Entrectinib dose that was taken prior to initiating the CYP3A inhibitor.
Swallow capsules whole. Do not crush or chew the capsules.
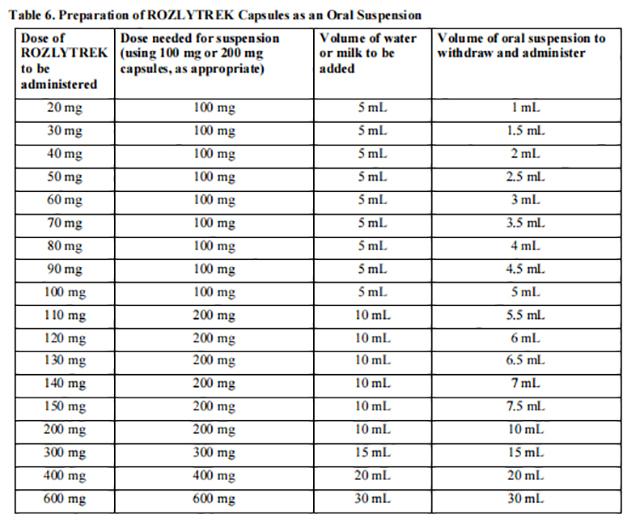
It is recommended that a healthcare provider discuss with the patient or caregiver, the volume of water or milk to be added and oral suspension to withdraw, prior to administration of the first dose (see Table 6).
Table 6 provides the Entrectinib dose and volume of room temperature drinking water or milk required to prepare an oral suspension. Instruct patients or caregivers to carefully open capsule(s) and pour the contents into room temperature drinking water or milk to prepare an oral suspension. Let sit for 15 minutes.
Administer Entrectinib oral suspension immediately after preparation.
Discard any unused suspension if not used within 2 hours.
Instruct patients to drink water after taking the oral suspension to ensure Entrectinib has been completely swallowed.
If enteral administration (e.g., gastric or nasogastric tube) is required, administer the oral suspension via the tube. Use an enteral tube that is 8 FR or higher to administer dosing volumes of 3 mL or higher. Instruct patients to divide dosing volumes of 3 mL or higher into at least two aliquots and flush the tube after each administration. Flush the tube with a volume of water or milk that is equal to the aliquot administered. For a dose volume of 30 mL, divide into at least three (10 mL) aliquots. The tube should be flushed with water or milk after delivering each aliquot of Entrectinib.
Refer to the Instructions for Use for detailed instructions on preparation and administration of Entrectinib capsules as an oral suspension via an enteral tube.
Sprinkle pellets on one or more spoonfuls of a soft food (e.g., applesauce, yogurt, or pudding) and take within 20 minutes of preparation. Do not crush or chew to avoid a bitter taste.
The patient should drink water after taking the pellets to ensure the drug has been completely swallowed.
Do not attempt to use partial quantities of pellets from 50 mg pellet packets to prepare a dose.
Do not use the pellet formulation for enteral tube administration as the pellets may clog the tube.
Refer to the Instructions for Use for detailed instructions on preparation and administration of Entrectinib oral pellets.
from FDA,2024.01