Adults: The efficacy and safety of Eltrombopag in adult patients with persistent or chronic ITP were evaluated in three randomized, double-blind, placebo-controlled trials and in an open-label extension trial.
In Study TRA100773B and Study TRA100773A (referred to as Study 773B and Study 773A, respectively [NCT00102739]), patients who had completed at least one prior ITP therapy and who had a platelet count less than 30 x 109 /L were randomized to receive either Eltrombopag or placebo daily for up to 6 weeks, followed by 6 weeks off therapy. During the trials, Eltrombopag or placebo was discontinued if the platelet count exceeded 200 x 109 /L.
The median age of the patients was 50 years and 60% were female. Approximately 70% of the patients had received at least 2 prior ITP therapies (predominantly corticosteroids, immunoglobulins, rituximab, cytotoxic therapies, danazol, and azathioprine) and 40% of the patients had undergone splenectomy. The median baseline platelet counts (approximately 18 x 109 /L) were similar among all treatment groups.
Study 773B randomized 114 patients (2:1) to Eltrombopag 50 mg or placebo. Of 60 patients with documented time since diagnosis, approximately 17% met the definition of persistent ITP with time since diagnosis of 3-12 months. Study 773A randomized 117 patients (1:1:1:1) among placebo or 1 of 3 dose regimens of Eltrombopag, 30 mg, 50 mg, or 75 mg each administered daily. Of 51 patients with documented time since diagnosis, approximately 14% met the definition of persistent ITP.
The efficacy of Eltrombopag in this trial was evaluated by response rate, defined as a shift from a baseline platelet count of less than 30 x 109 /L to greater than or equal to 50 x 109 /L at any time during the treatment period (Table 16).
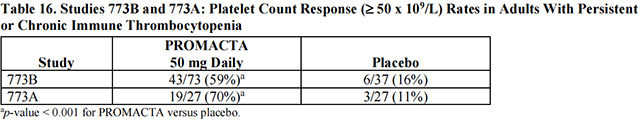
The platelet count response to Eltrombopag was similar among patients who had or had not undergone splenectomy. In general, increases in platelet counts were detected 1 week following initiation of Eltrombopag and the maximum response was observed after 2 weeks of therapy. In the placebo and 50-mg–dose groups of Eltrombopag, the trial drug was discontinued due to an increase in platelet counts to greater than 200 x 109 /L in 3% and 27% of the patients, respectively. The median duration of treatment with the 50-mg dose of Eltrombopag was 43 days in Study 773B and 42 days in Study 773A.
Of 7 patients who underwent hemostatic challenges, additional ITP medications were required in 3 of 3 placebo group patients and 0 of 4 patients treated with Eltrombopag. Surgical procedures accounted for most of the hemostatic challenges. Hemorrhage requiring transfusion occurred in one placebo group patient and no patients treated with Eltrombopag.
In the RAISE study (NCT00370331), 197 patients were randomized (2:1) to receive either Eltrombopag 50 mg once daily (n = 135) or placebo (n = 62) for 6 months, during which time the dose of Eltrombopag could be adjusted based on individual platelet counts. Of 145 patients with documented time since diagnosis, 19% met the definition of persistent ITP. Patients were allowed to taper or discontinue concomitant ITP medications after being treated with Eltrombopag for 6 weeks. Patients were permitted to receive rescue treatments at any time during the trial as clinically indicated.
The median ages of the patients treated with Eltrombopag and placebo were 47 years and 52.5 years, respectively. Approximately half of the patients treated with Eltrombopag and placebo (47% and 50%, respectively) were receiving concomitant ITP medication (predominantly corticosteroids) at randomization and had baseline platelet counts less than or equal to 15 x 109 /L (50% and 48%, respectively). A similar percentage of patients treated with Eltrombopag and placebo (37% and 34%, respectively) had a prior splenectomy.
The efficacy of Eltrombopag in this trial was evaluated by the odds of achieving a platelet count greater than or equal to 50 x 109 /L and less than or equal to 400 x 109 /L for patients receiving Eltrombopag relative to placebo and was based on patient response profiles throughout the 6-month treatment period. In 134 patients who completed 26 weeks of treatment, a sustained platelet response (platelet count greater than or equal to 50 x 109 /L and less than or equal to 400 x 109 /L for 6 out of the last 8 weeks of the 26-week treatment period in the absence of rescue medication at any time) was achieved by 60% of patients treated with Eltrombopag, compared with 10% of patients treated with placebo (splenectomized patients: Eltrombopag 51%, placebo 8%; non-splenectomized patients: Eltrombopag 66%, placebo 11%). The proportion of responders in the group of patients treated with Eltrombopag was between 37% and 56% compared with 7% and 19% in the placebo treatment group for all on-therapy visits. Patients treated with Eltrombopag were significantly more likely to achieve a platelet count between 50 x 109 /L and 400 x 109 /L during the entire 6-month treatment period compared with those patients treated with placebo.
Outcomes of treatment are presented in Table 17 for all patients enrolled in the trial.

Among 94 patients receiving other ITP therapy at baseline, 37 (59%) of 63 patients treated with Eltrombopag and 10 (32%) of 31 patients in the placebo group discontinued concomitant therapy at some time during the trial.
In the EXTEND study (NCT00351468), patients who completed any prior clinical trial with Eltrombopag were enrolled in an open-label, single-arm trial in which attempts were made to decrease the dose or eliminate the need for any concomitant ITP medications. Eltrombopag was administered to 302 patients in EXTEND; 218 patients completed 1 year, 180 patients completed 2 years, 107 patients completed 3 years, 75 patients completed 4 years, 34 patients completed 5 years, and 18 patients completed 6 years of therapy. The median baseline platelet count was 19 x 109 /L prior to administration of Eltrombopag. Median platelet counts at 1, 2, 3, 4, 5, 6, and 7 years on study were 85 x 109 /L, 85 x 109 /L, 105 x 109 /L, 64 x 109 /L, 75 x 109 /L, 119 x 109 /L, and 76 x 109 /L, respectively.
from FDA,2021.10
To investigate the role of eltrombopag in the treatment of specific diseases and···【more】
Release date:2025-03-21Recommended:225
To explore the performance of eltrombopag in the treatment of specific diseases ···【more】
Release date:2025-03-20Recommended:182
To explore the performance of eltrombopag in the treatment of specific diseases,···【more】
Release date:2025-03-20Recommended:181