The efficacy and safety of Daprodustat were evaluated as co-primary endpoints: the mean change in hemoglobin from baseline to the Evaluation Period (Weeks 28 to 52) and time to first adjudicated MACE (defined as all-cause mortality, non-fatal myocardial infarction, or non-fatal stroke), using a non-inferiority comparison to rhEPO (epoetin alfa and darbepoetin alfa) for both endpoints.
The lower limit of the 95% confidence interval (CI) for the overall hemoglobin treatment difference was greater than the pre-specified non-inferiority margin of -0.75 g/dL, demonstrating non-inferiority of Daprodustat to rhEPO with respect to the mean change in hemoglobin between baseline and over the Evaluation Period (see Table 6). Results were similar in patients receiving either hemodialysis or peritoneal dialysis.
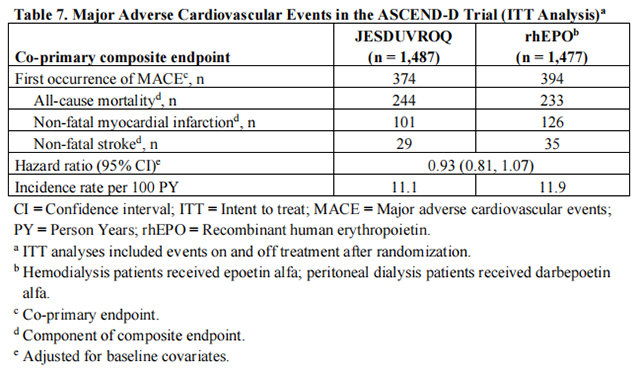
The hazard ratio for the time to first occurrence of MACE, a composite of all-cause mortality, non-fatal myocardial infarction, and non-fatal stroke, comparing Daprodustat to rhEPO was 0.93 (95% CI 0.81, 1.07) (Table 7). Non-inferiority of Daprodustat to rhEPO on MACE was achieved because the upper limit of the 95% CI for the MACE hazard ratio was less than the pre-specified non-inferiority margin of 1.25.
from FDA,2023.02