Correct and exclude other causes of anemia (e.g., vitamin deficiency, metabolic or chronic inflammatory conditions, bleeding) before initiating Daprodustat. Evaluate the iron status in all patients before and during treatment with Daprodustat. Administer supplemental iron therapy when serum ferritin is less than 100 mcg/mL or when serum transferrin saturation is less than 20%. The majority of patients with CKD will require supplemental iron during the course of therapy.
Assess serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and total bilirubin prior to initiation of Daprodustat. Repeat the liver tests if the patient develops signs or symptoms that could be consistent with liver disease during treatment with Daprodustat.
Individualize dosing and use the lowest dose of Daprodustat sufficient to reduce the need for red blood cell transfusions. Do not target a hemoglobin higher than 11 g/dL.
Daprodustat can be taken with or without food, and without regard to concomitant administration of iron or phosphate binders.
Daprodustat should be swallowed whole. Tablets should not be cut, crushed, or chewed.
Daprodustat can be administered without regard to the timing or type of dialysis.
If a dose of Daprodustat is missed, it should be taken as soon as possible, unless it is the same day as the next dose. In this case, the missed dose should be skipped, and the next dose taken at the usual time. Double-doses should not be taken to make-up for a missed dose.
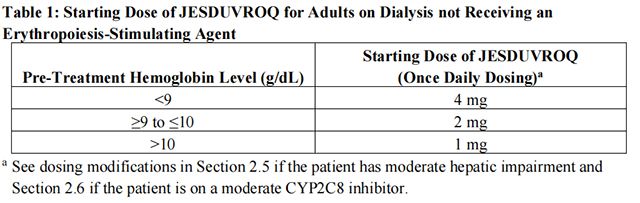
Adults Not Being Treated with an ESA: For adults not being treated with an ESA, the starting dose of Daprodustat is based on the hemoglobin level (see Table 1). Dose modifications are needed for patients receiving concomitant treatment with a moderate CYP2C8 inhibitor or moderate hepatic impairment.
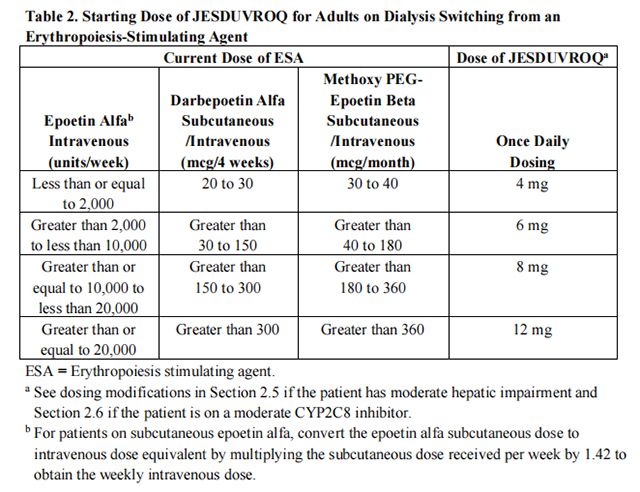
Adults Being Switched from an ESA: For adults being switched from an ESA to Daprodustat, the starting dose of Daprodustat is based on the dose regimen of the ESA at the time of substitution (see Table 2). Dose modifications are needed for patients receiving concomitant treatment with a moderate CYP2C8 inhibitor or moderate hepatic impairment.
Following initiation of therapy and after each dose adjustment, monitor hemoglobin every 2 weeks for the first month and then every 4 weeks thereafter.
When adjusting doses of Daprodustat, consider hemoglobin rate of rise, rate of decline and hemoglobin variability. Do not increase the dose of Daprodustat more frequently than once every 4 weeks.
• If the dose of Daprodustat needs to be adjusted, increase or decrease by one dose level at a time (see Table 3).
• Decrease the dose of Daprodustat if hemoglobin increases rapidly (e.g., greater than 1 g/dL over 2 weeks or greater than 2 g/dL over 4 weeks) or if the hemoglobin exceeds 11 g/dL.
• If hemoglobin exceeds 12 g/dL, interrupt treatment with Daprodustat. When the hemoglobin is within the target range, treatment may be restarted at one dose level lower (see Table 3).
• Treatment with Daprodustat should not be continued beyond 24 weeks of therapy if a clinically meaningful increase in hemoglobin level is not achieved. Alternative explanations for an inadequate response should be sought and treated before re-starting therapy.
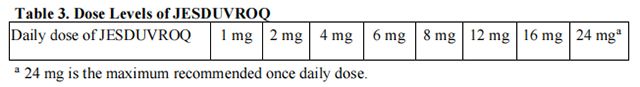
Reduce the starting dose of Daprodustat by half (see Tables 1 and 2) in patients with moderate hepatic impairment (Child-Pugh Class B) except in patients whose starting dose is already 1 mg.
Use of Daprodustat in patients with severe hepatic impairment (Child-Pugh Class C) is not recommended.
Reduce the starting dose of Daprodustat by half (see Tables 1 and 2) in patients who are on clopidogrel or a moderate CYP2C8 inhibitor except in patients whose starting dose is already 1 mg.
Monitor hemoglobin and adjust the dose of Daprodustat when initiating or stopping therapy with clopidogrel or a moderate CYP2C8 inhibitor during treatment with Daprodustat.
from FDA,2023.02