In Trial 1, the safety and efficacy of Dabrafenib were demonstrated in an international, multi center, randomized (3:1), open-label, active-controlled trial conducted in 250 patients with previously untreated BRAF V600E mutation-positive, unresectable or metastatic melanoma. Patients with any prior use of BRAF inhibitors or MEK inhibitors were excluded. Patients were randomized to receive Dabrafenib 150 mg by mouth twice daily (n = 187) or dacarbazine 1,000 mg/m2 intravenously every 3 weeks (n = 63). Randomization was stratified by disease stage at baseline. The main efficacy outcome measure was progression-free survival (PFS) as assessed by the investigator. In addition, an independent radiology review committee (IRRC) assessed the following efficacy outcome measures in pre specified supportive analyses: PFS, confirmed objective response rate (ORR), and duration of response.
The median age of patients in Trial 1 was 52 years. The majority of the trial population was male (60%), white (99%), had an ECOG performance status of 0 (67%), M1c disease (66%), and normal LDH (62%). All patients had tumor tissue with mutations in BRAF V600E as determined by a clinical trial assay at a centralized testing site. Tumor samples from 243 patients (97%) were tested retrospectively, using an FDA-approved companion diagnostic test, THxID™-BRAF assay.
The median duration of follow-up prior to initiation of alternative treatment in the Dabrafenib arm was 5.1 months and in the dacarbazine arm was 3.5 months. Twenty-eight (44%) patients crossed over from the dacarbazine arm at the time of disease progression to receive Dabrafenib.
Trial 1 demonstrated a statistically significant increase in progression-free survival in the patients treated with Dabrafenib. Table 5 and Figure 1 summarize the PFS results.
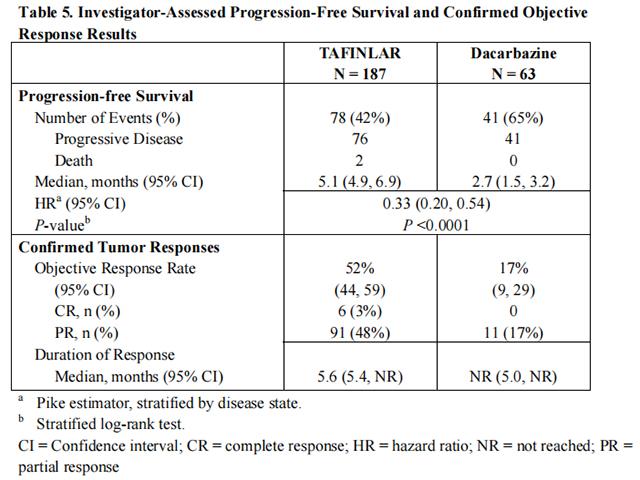
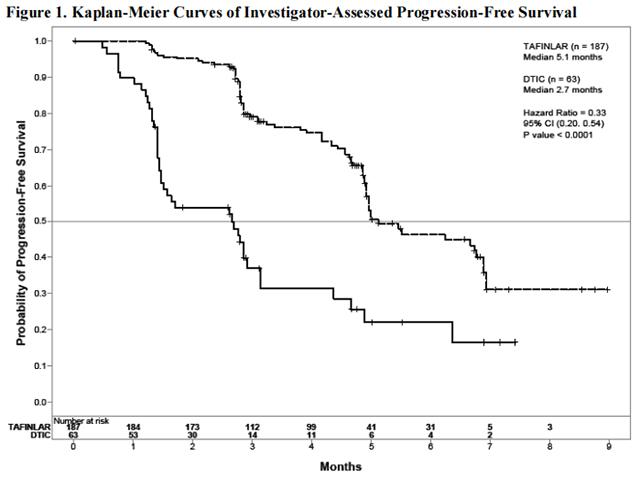
In supportive analyses based on IRRC assessment and in an exploratory subgroup analysis of patients with retrospectively confirmed V600E mutation-positive melanoma with the THxID™ BRAF assay, the PFS results were consistent with those of the primary efficacy analysis.
The activity of Dabrafenib for the treatment of BRAF V600E mutation-positive melanoma, metastatic to the brain was evaluated in a single arm, open-label, two-cohort, multi-center trial (Trial 2). All patients received Dabrafenib 150 mg twice daily. Patients in Cohort A (n=74) had received no prior local therapy for brain metastases, while patients in Cohort B (n=65) had received at least one local therapy for brain metastases, including, but not limited to, surgical resection, whole brain radiotherapy, or stereotactic radiosurgery such as gamma knife, linear accelerated-based radiosurgery, charged particles, or CyberKnife. In addition, patients in Cohort B were required to have evidence of disease progression in a previously treated lesion or an untreated lesion. Additional eligibility criteria were at least one measurable lesion of 0.5 cm or greater in largest diameter on contrast-enhanced MRI, stable or decreasing corticosteroid dose, and no more than two prior systemic regimens for treatment of metastatic disease. The primary outcome measure was estimation of the overall intracranial response rate (OIRR) in each cohort.
The median age of patients in Cohort A was 50 years, 72% were male, 100% were white, 59% had a pre-treatment ECOG performance status of 0, and 57% had an elevated LDH value at baseline. The median age of patients in Cohort B was 51 years, 63% were male, 98% were white, 66% had a pre-treatment ECOG performance status of 0, and 54% had an elevated LDH value at baseline. Efficacy results as determined by an independent radiology review committee, masked to investigator response assessments, are provided in Table 6.
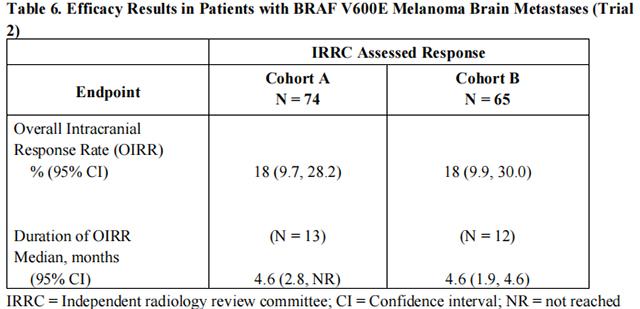
from FDA,2013.05