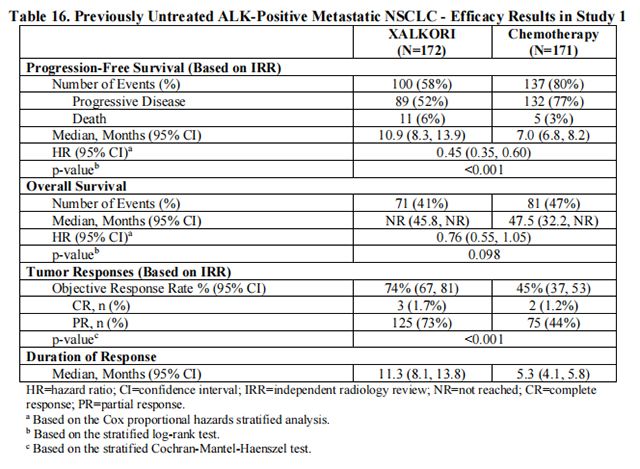
The efficacy of Crizotinib for the treatment of patients with ALK-positive metastatic NSCLC, who had not received previous systemic treatment for advanced disease, was demonstrated in a randomized, multicenter, open-label, active-controlled study (Study 1).
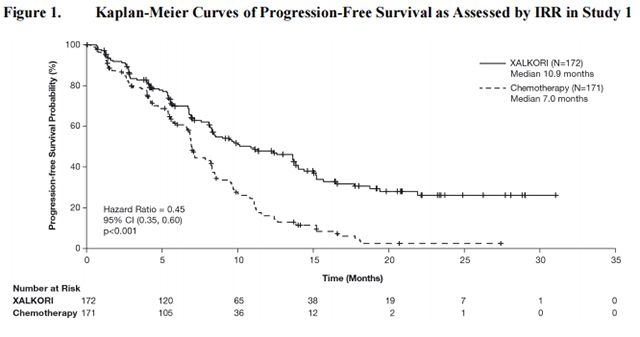
Study 1 demonstrated a statistically significant improvement in PFS in patients treated with Crizotinib. There was no statistically significant difference in OS between patients treated with Crizotinib and patients treated with chemotherapy. Table 16 and Figure 1 summarize the efficacy results. Exploratory patient-reported symptom measures of baseline and post-treatment dyspnea, cough, and chest pain suggested a delay in time to development of or worsening of dyspnea, but not cough or chest pain, in patients treated with Crizotinib as compared to chemotherapy. The patient-reported delay in onset or worsening of dyspnea may be an overestimation because patients were not blinded to treatment assignment.
from FDA,2023.09
Assessments of the medical value of specific treatment options need to be based ···【more】
Release date:2025-04-03Recommended:223
The effect of treatment is a key indicator for evaluating the drug.Is crizotinib···【more】
Release date:2025-04-03Recommended:184