Select patients for treatment of metastatic NSCLC with Ceritinib based on the presence of ALK positivity in tumor specimens.
The recommended dosage of Ceritinib is 450 mg orally once daily with food until disease progression or unacceptable toxicity.
If a dose of Ceritinib is missed, make up that dose unless the next dose is due within 12 hours.
If vomiting occurs during the course of treatment, do not administer an additional dose and continue with the next scheduled dose of Ceritinib.
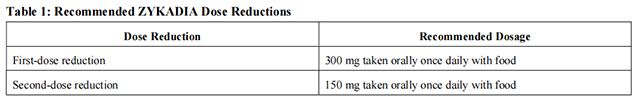
Discontinue Ceritinib for patients unable to tolerate 150 mg taken orally once daily with food.
Dosage modifications for selected adverse reactions of Ceritinib are provided in Table 2. If dose reduction is required due to an adverse reaction not listed in Table 2, then reduce the daily dose of Ceritinib by 150 mg.
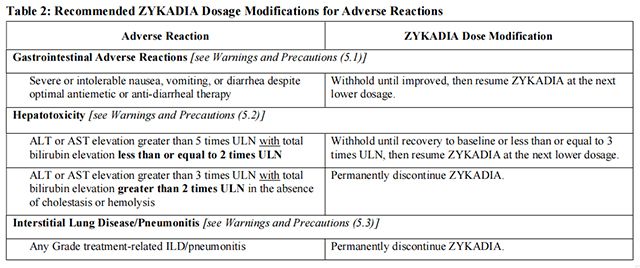
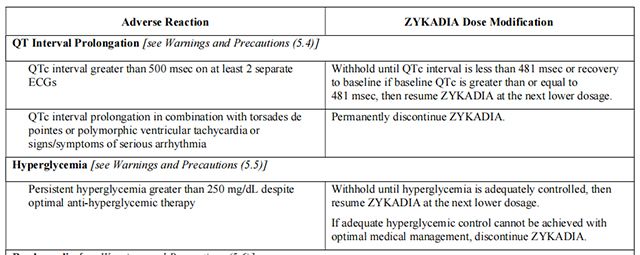
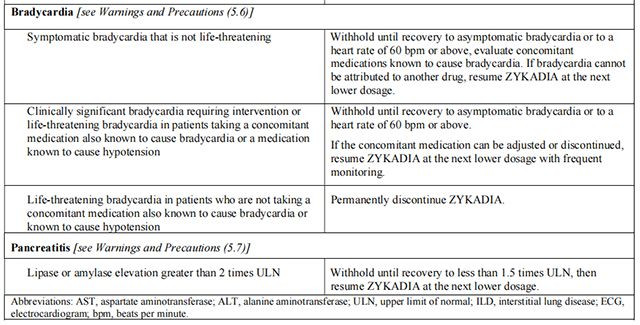
Avoid concurrent use of strong CYP3A inhibitors during treatment with Ceritinib.
If concurrent use of a strong CYP3A inhibitor is unavoidable, reduce the Ceritinib dose by approximately one-third, rounded to the nearest multiple of the 150 mg dosage strength. After discontinuation of a strong CYP3A inhibitor, resume the Ceritinib dose that was taken prior to initiating the strong CYP3A inhibitor.
For patients with severe hepatic impairment (Child-Pugh C), reduce the Ceritinib dose by approximately one-third, rounded to the nearest multiple of the 150 mg dosage strength.
from FDA,2021.10