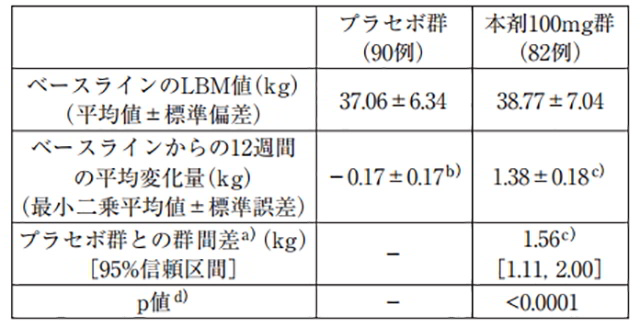
173 cases of non-small cell lung cancer in Japanese patients (Note 1)Among the targets, 83 cases and 90 cases were included, and our 100mg sample was also included in the sample.Administer orally once a day on an empty stomach for 12 weeks. Main evaluation projects.
The average weight gain over a period of 12 weeks excluding body fat (LBM) is compared to the following:Intentional bias (p<0.0001) 19). ま, Safety evaluation.Out of 83 cases, 34 cases (41.0%) had side effects (clinical examination value).Abnormalities are detected. Main and Side Effects: First degree atrioventricular injury.There were 5 cases (6.0%) of rash and 5 cases (6.0%) of rash.
fromJP,2023.02
In 2021, Japan approved Anamorelin for the treatment of cachexia caused by vario···【more】
Release date:2024-08-21Recommended:257
Anamorelin is a novel ghrelin receptor agonist specifically designed for the tre···【more】
Release date:2024-08-21Recommended:328
Anamorelin is a selective oral medication that increases a patient's appetit···【more】
Release date:2024-08-21Recommended:239