The efficacy of Alectinib for the adjuvant treatment of patients with ALK-positive NSCLC following complete tumor resection was evaluated in a global, randomized open-label clinical trial (ALINA: NCT03456076).
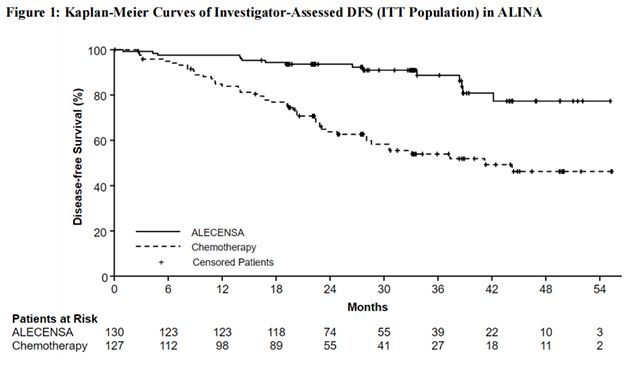
ALINA demonstrated a statistically significant improvement in DFS for patients treated with Alectinib compared to patients treated with chemotherapy. OS data were not mature at the time of DFS analysis with 2.3% of deaths reported in the ITT population.
The efficacy results from ALINA are summarized in Table 10 and Figure 1.
In an exploratory analysis of site(s) of relapse, the proportion of patients with brain involvement at the time of disease recurrence was 4 patients (3.1%) in the Alectinib arm and 14 patients (11%) in the chemotherapy arm.
from FDA,2024.04