Select patients with resectable tumors for the adjuvant treatment of NSCLC with Alectinib based on the presence of ALK positivity in tumor tissue.
Select patients for the treatment of metastatic NSCLC with Alectinib based on the presence of ALK positivity in tumor tissue or plasma specimens. If ALK rearrangements are not detected in a plasma specimen, test tumor tissue if feasible.
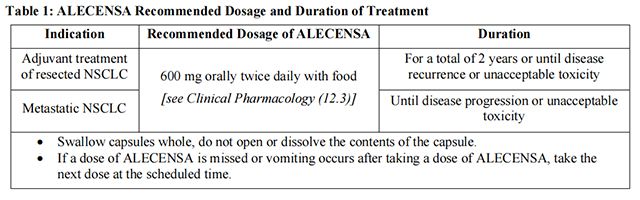
The recommended dosage information for Alectinib is provided in Table 1.
The recommended dose of Alectinib in patients with severe hepatic impairment (Child-Pugh C) is 450 mg orally twice daily.
The dose reduction schedule for Alectinib is provided in Table 2.
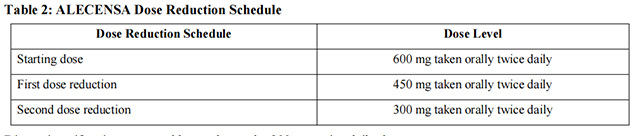
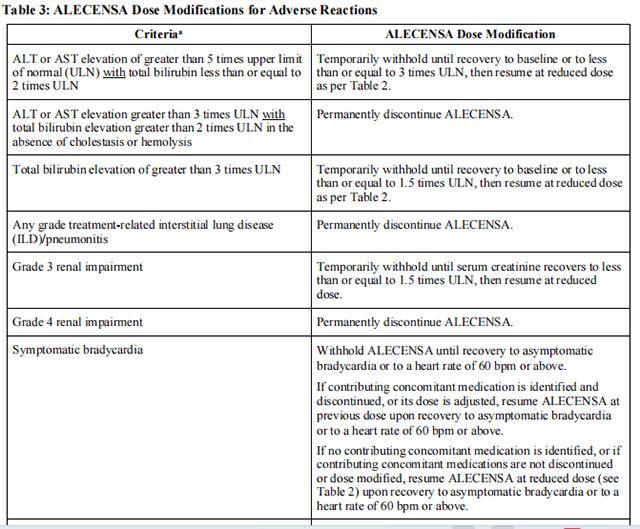
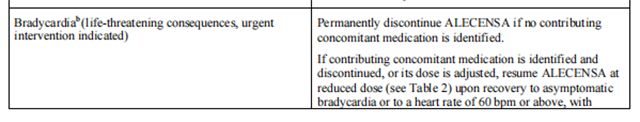
Discontinue if patients are unable to tolerate the 300 mg twice daily dose. Recommendations for dose modifications of Alectinib in case of adverse reactions are provided in Table 3.
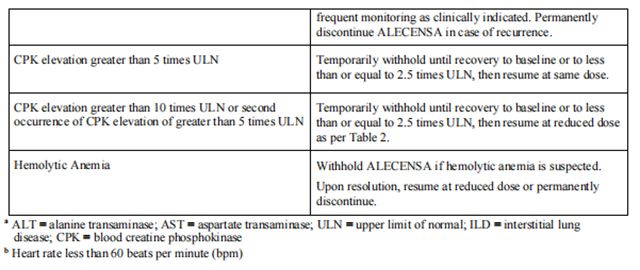
from FDA,2024.04