

Another Name拉罗替尼、LOXO101、Laronib、Vitrakvi
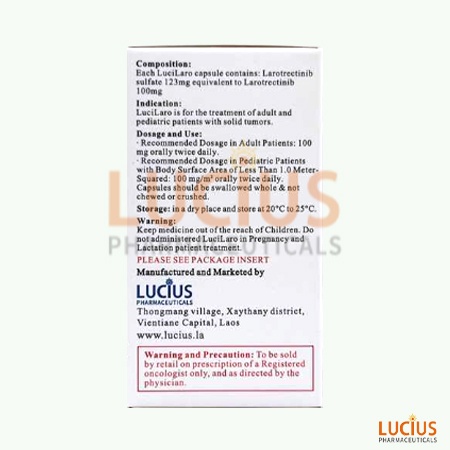
IndicationsLarotrectinib is indicated for the treatment of adult and pediatric patients with solid tumors.
Reg No.05 L 0977/23
Inspection NO.
On November 26, 2018, the FDA approved the marketing of Larotrectinib for the treatment of adult and pediatric solid tumor patients carrying NTRK gene fusion.
The therapeutic effect of Larotrectinib has been validated, and most patients have improved their condition after using Larotrectinib.
Larotrectinib is the world's first TRK inhibitor approved for the treatment of NTRK gene fusion patients. The original intention of its development is based on the concept of precision medicine, targeting tumors with specific molecular characteristics for treatment, namely NTRK gene fusion.
Larotrectinib
Larotrectinib is suitable for the treatment of solid tumor patients in adults and children.
Based on literature reports in human subjects with congenital mutations leading to changes in TRK signaling, findings from animal studies, and its mechanism of action, Larotrectinib can cause embryo-fetal harm when administered to a pregnant woman. There are no available data on Larotrectinib use in pregnant women. Advise pregnant women of the potential risk to a fetus.
There are no data on the presence of Larotrectinib or its metabolites in human milk and no data on its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with
Larotrectinib and for 1 week after the final dose.
Verify pregnancy status in females of reproductive potential prior to initiating Larotrectinib.Larotrectinib can cause embryo-fetal harm when administered to a pregnant woman.Advise female patients of reproductive potential to use effective contraception during treatment with Larotrectinib and for at least 1 week after the final dose.
Males
Advise males with female partners of reproductive potential to use effective contraception during treatment with Larotrectinib and for 1 week after the final dose.
Females
Based on histopathological findings in the reproductive tracts of female rats in a 1-month repeated-dose study, Larotrectinib may reduce fertility.
Pediatric patients need to use it under the guidance of a doctor.
Of 279 patients in the overall safety population who received Larotrectinib, 19% of patients were ≥ 65 years of age and 5% of patients were ≥ 75 years of age. Clinical studies of Larotrectinib did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A). Larotrectinib clearance was reduced in subjects with moderate (Child-Pugh B) to severe (Child-Pugh C) hepatic impairment. Reduce Larotrectinib dose as recommended.
No dose adjustment is recommended for patients with renal impairment of any severity.
Drug overdose is not yet clear.
Store capsules at room temperature 20°C to 25°C (68°F to 77°F); temperature excursions between 15°C and 30°C (59°F to 86°F) are permitted.
The mean absolute bioavailability of Larotrectinib capsules was 34% (range: 32% to 37%). In healthy subjects, the AUC of Larotrectinib oral solution was similar to that of the capsules and the Cmax was 36% higher with the oral solution.
from FDA,2022.11